当前位置:
X-MOL 学术
›
Asian J. Pharm. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting the organelle for radiosensitization in cancer radiotherapy
Asian Journal of Pharmaceutical Sciences ( IF 10.7 ) Pub Date : 2024-03-10 , DOI: 10.1016/j.ajps.2024.100903 Xiaoyan Sun 1, 2 , Linjie Wu 1, 2 , Lina Du 3 , Wenhong Xu 4 , Min Han 1, 2, 4, 5, 6
Asian Journal of Pharmaceutical Sciences ( IF 10.7 ) Pub Date : 2024-03-10 , DOI: 10.1016/j.ajps.2024.100903 Xiaoyan Sun 1, 2 , Linjie Wu 1, 2 , Lina Du 3 , Wenhong Xu 4 , Min Han 1, 2, 4, 5, 6
Affiliation
|
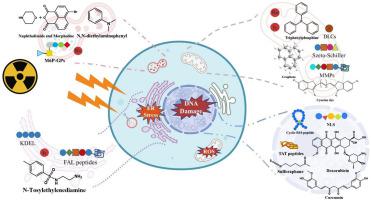
Radiotherapy is a well-established cytotoxic therapy for local solid cancers, utilizing high-energy ionizing radiation to destroy cancer cells. However, this method has several limitations, including low radiation energy deposition, severe damage to surrounding normal cells, and high tumor resistance to radiation. Among various radiotherapy methods, boron neutron capture therapy (BNCT) has emerged as a principal approach to improve the therapeutic ratio of malignancies and reduce lethality to surrounding normal tissue, but it remains deficient in terms of insufficient boron accumulation as well as short retention time, which limits the curative effect. Recently, a series of radiosensitizers that can selectively accumulate in specific organelles of cancer cells have been developed to precisely target radiotherapy, thereby reducing side effects of normal tissue damage, overcoming radioresistance, and improving radiosensitivity. In this review, we mainly focus on the field of nanomedicine-based cancer radiotherapy and discuss the organelle-targeted radiosensitizers, specifically including nucleus, mitochondria, endoplasmic reticulum and lysosomes. Furthermore, the organelle-targeted boron carriers used in BNCT are particularly presented. Through demonstrating recent developments in organelle-targeted radiosensitization, we hope to provide insight into the design of organelle-targeted radiosensitizers for clinical cancer treatment.
中文翻译:

癌症放射治疗中靶向细胞器的放射增敏
放射疗法是针对局部实体癌的一种成熟的细胞毒性疗法,利用高能电离辐射来破坏癌细胞。然而,这种方法有一些局限性,包括辐射能量沉积低、对周围正常细胞的严重损伤以及肿瘤对辐射的抵抗力高。在各种放疗方法中,硼中子俘获疗法(BNCT)已成为提高恶性肿瘤治疗率、降低对周围正常组织杀伤力的主要方法,但仍存在硼积累不足、保留时间短等问题。从而限制了疗效。近年来,人们开发出一系列能够选择性积累在癌细胞特定细胞器中的放射增敏剂,以精确靶向放射治疗,从而减少正常组织损伤的副作用,克服放射抗性,提高放射敏感性。在这篇综述中,我们主要关注基于纳米医学的癌症放射治疗领域,讨论细胞器靶向的放射增敏剂,具体包括细胞核、线粒体、内质网和溶酶体。此外,还特别介绍了 BNCT 中使用的细胞器靶向硼载体。通过展示细胞器靶向放射增敏的最新进展,我们希望为临床癌症治疗的细胞器靶向放射增敏剂的设计提供见解。
更新日期:2024-03-10
中文翻译:

癌症放射治疗中靶向细胞器的放射增敏
放射疗法是针对局部实体癌的一种成熟的细胞毒性疗法,利用高能电离辐射来破坏癌细胞。然而,这种方法有一些局限性,包括辐射能量沉积低、对周围正常细胞的严重损伤以及肿瘤对辐射的抵抗力高。在各种放疗方法中,硼中子俘获疗法(BNCT)已成为提高恶性肿瘤治疗率、降低对周围正常组织杀伤力的主要方法,但仍存在硼积累不足、保留时间短等问题。从而限制了疗效。近年来,人们开发出一系列能够选择性积累在癌细胞特定细胞器中的放射增敏剂,以精确靶向放射治疗,从而减少正常组织损伤的副作用,克服放射抗性,提高放射敏感性。在这篇综述中,我们主要关注基于纳米医学的癌症放射治疗领域,讨论细胞器靶向的放射增敏剂,具体包括细胞核、线粒体、内质网和溶酶体。此外,还特别介绍了 BNCT 中使用的细胞器靶向硼载体。通过展示细胞器靶向放射增敏的最新进展,我们希望为临床癌症治疗的细胞器靶向放射增敏剂的设计提供见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号