当前位置:
X-MOL 学术
›
Chemosphere
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption effects of electron scavengers and inorganic ions on catalysts for catalytic oxidation of sulfamethoxazole in radiation treatment
Chemosphere ( IF 8.1 ) Pub Date : 2024-03-12 , DOI: 10.1016/j.chemosphere.2024.141675 Kang Lee 1 , Tae-Hun Kim 2 , Sang-Hee Jo 2 , Seungho Yu 2
Chemosphere ( IF 8.1 ) Pub Date : 2024-03-12 , DOI: 10.1016/j.chemosphere.2024.141675 Kang Lee 1 , Tae-Hun Kim 2 , Sang-Hee Jo 2 , Seungho Yu 2
Affiliation
|
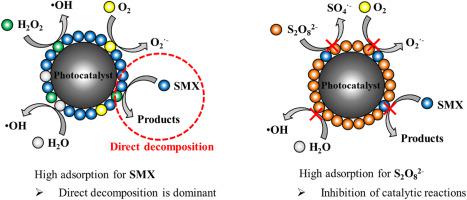
This study aimed to investigate adsorption effects of electron scavengers (HO and SO) on oxidation performance for mineralization of sulfamethoxazole (SMX) in radiation treatment using catalysts (AlO, TiO). Hydrogen peroxide (HO, 1 mM) as an electron scavenger showed weak adsorption onto catalysts (0.012 mmol g-AlO and 0.004 mmol g-TiO, respectively), leading to an increase in TOC removal efficiency of SMX within the absorbed dose of 30 kGy by 12.3% with AlO and by 8.0% with TiO. The weak adsorption of HO onto the catalyst allowed it to act as an electron scavenger, promoting indirect decomposition reactions. However, high adsorption of SO (1 mM) onto AlO (0.266 mmol g-AlO) showed a decrease in TOC removal efficiency of SMX from 76.2% to 30.2% within the absorbed dose of 30 kGy. The high adsorption of SO onto the catalyst inhibited direct decomposition reaction by reducing adsorption of SMX on catalysts. TOC removal efficiency for AlO without electron scavengers in an acidic condition was higher than that in a neutral or alkaline condition. However, TOC removal efficiency for AlO with SO was higher in a neutral condition than in other pH conditions. This indicates that the pH of a solution plays a critical role in the catalytic oxidation performance by determining surface charges of catalysts and yield of reactive radicals produced from water radiolysis. In the radiocatalytic system, HO enhances the oxidation performance of catalysts (AlO and TiO) over a wide pH range (3–11). Meanwhile, SO is not suitable with AlO in acidic conditions because of its strong adsorption onto AlO in this study.
中文翻译:

辐射处理中电子清除剂和无机离子对磺胺甲恶唑催化氧化催化剂的吸附作用
本研究旨在研究电子清除剂(H2O和SO)对催化剂(Al2O、TiO)辐射处理中磺胺甲恶唑(SMX)矿化氧化性能的吸附作用。过氧化氢(H2O,1 mM)作为电子清除剂对催化剂(分别为 0.012 mmol g-Al2O 和 0.004 mmol g-TiO)表现出弱吸附,导致 SMX 在 30 kGy 吸收剂量内的 TOC 去除效率提高Al2O 降低 12.3%,TiO 降低 8.0%。 H2O在催化剂上的微弱吸附使其能够充当电子清除剂,促进间接分解反应。然而,SO (1 mM) 在 Al2O (0.266 mmol g-Al2O) 上的高吸附表明,在 30 kGy 的吸收剂量内,SMX 的 TOC 去除效率从 76.2% 降低到 30.2%。 SO在催化剂上的高吸附通过减少SMX在催化剂上的吸附来抑制直接分解反应。没有电子清除剂的 Al2O3 在酸性条件下的 TOC 去除效率高于中性或碱性条件下的 TOC 去除效率。然而,在中性条件下,Al2O3 与 SO 的 TOC 去除效率高于其他 pH 条件。这表明溶液的 pH 值通过确定催化剂的表面电荷和水辐射分解产生的反应自由基的产量,在催化氧化性能中发挥着关键作用。在放射催化系统中,H2O 可以在较宽的 pH 范围 (3-11) 内增强催化剂(Al2O 和 TiO)的氧化性能。同时,在本研究中,由于SO对Al2O的强烈吸附,因此在酸性条件下不适合与Al2O一起使用。
更新日期:2024-03-12
中文翻译:

辐射处理中电子清除剂和无机离子对磺胺甲恶唑催化氧化催化剂的吸附作用
本研究旨在研究电子清除剂(H2O和SO)对催化剂(Al2O、TiO)辐射处理中磺胺甲恶唑(SMX)矿化氧化性能的吸附作用。过氧化氢(H2O,1 mM)作为电子清除剂对催化剂(分别为 0.012 mmol g-Al2O 和 0.004 mmol g-TiO)表现出弱吸附,导致 SMX 在 30 kGy 吸收剂量内的 TOC 去除效率提高Al2O 降低 12.3%,TiO 降低 8.0%。 H2O在催化剂上的微弱吸附使其能够充当电子清除剂,促进间接分解反应。然而,SO (1 mM) 在 Al2O (0.266 mmol g-Al2O) 上的高吸附表明,在 30 kGy 的吸收剂量内,SMX 的 TOC 去除效率从 76.2% 降低到 30.2%。 SO在催化剂上的高吸附通过减少SMX在催化剂上的吸附来抑制直接分解反应。没有电子清除剂的 Al2O3 在酸性条件下的 TOC 去除效率高于中性或碱性条件下的 TOC 去除效率。然而,在中性条件下,Al2O3 与 SO 的 TOC 去除效率高于其他 pH 条件。这表明溶液的 pH 值通过确定催化剂的表面电荷和水辐射分解产生的反应自由基的产量,在催化氧化性能中发挥着关键作用。在放射催化系统中,H2O 可以在较宽的 pH 范围 (3-11) 内增强催化剂(Al2O 和 TiO)的氧化性能。同时,在本研究中,由于SO对Al2O的强烈吸附,因此在酸性条件下不适合与Al2O一起使用。































 京公网安备 11010802027423号
京公网安备 11010802027423号