当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of a Fungal P450 with an Unusual Two-Step Mechanism for Constructing a Bicyclo[3.2.2]nonane Skeleton
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-14 , DOI: 10.1021/jacs.4c01284 Huibin Xu 1 , Zhenbo Yuan 1 , Sai Yang 1 , Zengping Su 1 , Xiao-Dong Hou 1 , Zhiwei Deng 1 , Yan Zhang 2 , Yijian Rao 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-14 , DOI: 10.1021/jacs.4c01284 Huibin Xu 1 , Zhenbo Yuan 1 , Sai Yang 1 , Zengping Su 1 , Xiao-Dong Hou 1 , Zhiwei Deng 1 , Yan Zhang 2 , Yijian Rao 1
Affiliation
|
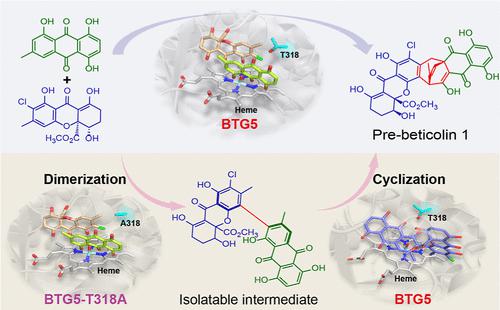
The successful biomimetic or chemoenzymatic synthesis of target natural products (NPs) and their derivatives relies on enzyme discovery. Herein, we discover a fungal P450 BTG5 that can catalyze the formation of a bicyclo[3.2.2]nonane structure through an unusual two-step mechanism of dimerization and cyclization in the biosynthesis of beticolin 1, whose bicyclo[3.2.2]nonane skeleton connects an anthraquinone moiety and a xanthone moiety. Further investigation reveals that BTG5-T318 not only determines the substrate selectivity but also alters the catalytic reactions, which allows the separation of the reaction to two individual steps, thereby understanding its catalytic mechanism. It reveals that the first heterodimerization undergoes the common oxidation process for P450s, while the second uncommon formal redox-neutral cyclization step is proved as a redox-mediated reaction, which has never been reported. Therefore, this work advances our understanding of P450-catalyzed reactions and paves the way for expansion of the diversity of this class of NPs through synthetic biology.
中文翻译:

发现真菌 P450 具有构建双环[3.2.2]壬烷骨架的不寻常的两步机制
目标天然产物(NP)及其衍生物的成功仿生或化学酶合成依赖于酶的发现。在此,我们发现了一种真菌P450 BTG5,它可以在甜菜碱1的生物合成中通过不寻常的二聚和环化两步机制催化双环[3.2.2]壬烷结构的形成,其双环[3.2.2]壬烷骨架连接蒽醌部分和呫吨酮部分。进一步的研究表明,BTG5-T318不仅决定底物选择性,还改变催化反应,从而将反应分离为两个单独的步骤,从而了解其催化机制。它揭示了第一个异二聚化经历了 P450 的常见氧化过程,而第二个不常见的正式氧化还原中性环化步骤被证明是氧化还原介导的反应,这从未被报道过。因此,这项工作增进了我们对 P450 催化反应的理解,并为通过合成生物学扩展此类 NP 的多样性铺平了道路。
更新日期:2024-03-14
中文翻译:

发现真菌 P450 具有构建双环[3.2.2]壬烷骨架的不寻常的两步机制
目标天然产物(NP)及其衍生物的成功仿生或化学酶合成依赖于酶的发现。在此,我们发现了一种真菌P450 BTG5,它可以在甜菜碱1的生物合成中通过不寻常的二聚和环化两步机制催化双环[3.2.2]壬烷结构的形成,其双环[3.2.2]壬烷骨架连接蒽醌部分和呫吨酮部分。进一步的研究表明,BTG5-T318不仅决定底物选择性,还改变催化反应,从而将反应分离为两个单独的步骤,从而了解其催化机制。它揭示了第一个异二聚化经历了 P450 的常见氧化过程,而第二个不常见的正式氧化还原中性环化步骤被证明是氧化还原介导的反应,这从未被报道过。因此,这项工作增进了我们对 P450 催化反应的理解,并为通过合成生物学扩展此类 NP 的多样性铺平了道路。

































 京公网安备 11010802027423号
京公网安备 11010802027423号