当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Subtle Structural Changes across the Boundary between A2AR/A2BR Dual Antagonism and A2BR Antagonism: A Novel Class of 2-Aminopyrimidine-Based Derivatives
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-03-14 , DOI: 10.1021/acs.jmedchem.4c00250
Haojie Wang 1 , Xinyu Yang 2 , Yan Li 1 , Shuyin Ze 3 , Bo Feng 4 , Yuan Weng 3 , Aoqi Gao 3 , Gaojie Song 3 , Mingyao Liu 3, 5 , Qiong Xie 1 , Yonghui Wang 1 , Weiqiang Lu 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-03-14 , DOI: 10.1021/acs.jmedchem.4c00250
Haojie Wang 1 , Xinyu Yang 2 , Yan Li 1 , Shuyin Ze 3 , Bo Feng 4 , Yuan Weng 3 , Aoqi Gao 3 , Gaojie Song 3 , Mingyao Liu 3, 5 , Qiong Xie 1 , Yonghui Wang 1 , Weiqiang Lu 2
Affiliation
|
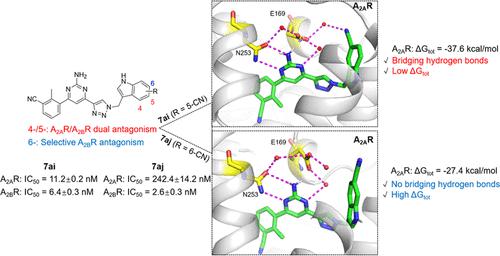
Aberrantly elevated adenosine in the tumor microenvironment exerts its immunosuppressive functions through adenosine receptors A2AR and A2BR. Antagonism of A2AR and A2BR has the potential to suppress tumor growth. Herein, we report a systemic assessment of the effects of an indole modification at position 4, 5, 6, or 7 on both A2AR/A2BR activity and selectivity of novel 2-aminopyrimidine compounds. Substituting indole at the 4-/5-position produced potent A2AR/A2BR dual antagonism, whereas the 6-position of indole substitution gave highly selective A2BR antagonism. Molecular dynamics simulation showed that the 5-cyano compound 7ai had a lower binding free energy than the 6-cyano compound 7aj due to water-bridged hydrogen bond interactions with E169 or F168 in A2AR. Of note, dual A2AR/A2BR antagonism by compound 7ai can profoundly promote the activation and cytotoxic function of T cells. This work provided a strategy for obtaining novel dual A2AR/A2BR or A2BR antagonists by fine-tuning structural modification.
中文翻译:

跨越 A2AR/A2BR 双重拮抗和 A2BR 拮抗之间边界的细微结构变化:一类基于 2-氨基嘧啶的新型衍生物
肿瘤微环境中异常升高的腺苷通过腺苷受体 A2AR 和 A2BR 发挥其免疫抑制功能。A2AR 和 A2BR 的拮抗作用有可能抑制肿瘤生长。在此,我们报告了第 4、5、6 或 7 位吲哚修饰对新型 2-氨基嘧啶化合物的 A2AR/A2BR 活性和选择性影响的系统评估。在 4-/5 位取代吲哚产生有效的 A2AR/A2BR 双重拮抗作用,而吲哚取代的 6 位产生高度选择性的 A2BR 拮抗作用。分子动力学模拟表明,由于在 A2AR 中与 E169 或 F168 发生水桥氢键相互作用,5-氰基化合物 7ai 的结合自由能低于 6-氰基化合物 7aj。值得注意的是,化合物 7ai 的双重 A2AR/A2BR 拮抗作用可以深度促进 T 细胞的活化和细胞毒功能。这项工作提供了一种通过微调结构修饰获得新型双重 A2AR/A2BR 或 A2BR 拮抗剂的策略。
更新日期:2024-03-14
中文翻译:

跨越 A2AR/A2BR 双重拮抗和 A2BR 拮抗之间边界的细微结构变化:一类基于 2-氨基嘧啶的新型衍生物
肿瘤微环境中异常升高的腺苷通过腺苷受体 A2AR 和 A2BR 发挥其免疫抑制功能。A2AR 和 A2BR 的拮抗作用有可能抑制肿瘤生长。在此,我们报告了第 4、5、6 或 7 位吲哚修饰对新型 2-氨基嘧啶化合物的 A2AR/A2BR 活性和选择性影响的系统评估。在 4-/5 位取代吲哚产生有效的 A2AR/A2BR 双重拮抗作用,而吲哚取代的 6 位产生高度选择性的 A2BR 拮抗作用。分子动力学模拟表明,由于在 A2AR 中与 E169 或 F168 发生水桥氢键相互作用,5-氰基化合物 7ai 的结合自由能低于 6-氰基化合物 7aj。值得注意的是,化合物 7ai 的双重 A2AR/A2BR 拮抗作用可以深度促进 T 细胞的活化和细胞毒功能。这项工作提供了一种通过微调结构修饰获得新型双重 A2AR/A2BR 或 A2BR 拮抗剂的策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号