当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel 5-Sulfonyl-1,3,4-thiadiazole-Substituted Flavonoids as Potential Bactericides and Fungicides: Design, Synthesis, Three-Dimensional Quantitative Structure–Activity Relationship Studies
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-03-14 , DOI: 10.1021/acs.jafc.3c06367 Peng Dai 1 , Jian Jiao 1 , Yufei Li 1 , Peng Teng 1 , Qingqing Wang 1 , Yuchuan Zhu 1 , Weihua Zhang 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-03-14 , DOI: 10.1021/acs.jafc.3c06367 Peng Dai 1 , Jian Jiao 1 , Yufei Li 1 , Peng Teng 1 , Qingqing Wang 1 , Yuchuan Zhu 1 , Weihua Zhang 1
Affiliation
|
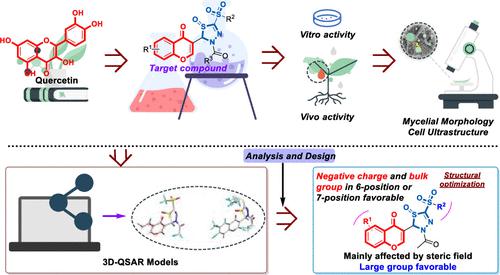
Flavonoids, ubiquitous natural products, provide sources for drug discovery owing to their structural diversity, broad-spectrum pharmacological activity, and excellent environmental compatibility. To develop antibacterial and antifungal agents with novel mechanisms of action and innovative structures, a series of novel 5-sulfonyl-1,3,4-thiadiazole-substituted flavonoids were designed and synthesized, and their biological activities against seven agriculturally common phytopathogenic microorganisms were evaluated. The results of the antimicrobial bioassay showed that most of the target compounds displayed excellent inhibitory effects against Xanthomonas oryzae, Rhizoctonia solani, and Colletotrichum orbiculare. Compounds 1, 3, 7, 9, 13, and 14 exhibited remarkable antibacterial activity against X. oryzae pv. oryzae with EC50 values below 10 μg/mL, which were superior to bismerthiazol (70.89 μg/mL). Compound 2 (EC50 = 0.41 μg/mL) displayed the most effective inhibitory potency against R. solani in vivo, comparable protective effects with the positive control carbendizam. Preliminary mechanistic studies indicated that compound 2 induced disordered entanglement of hyphae, shrinkage of hyphal surfaces, extravasation of cellular contents, and vacuole swelling and rupture, which disrupted normal hyphal growth. Subsequently, compounds 35–53 with good antifungal activity were designed and synthesized based on reliable three-dimensional quantitative structure–activity relationship (3D-QSAR) models. Compound 49 showed high efficacy and superior antifungal activity against R. solani, with an EC50 value of 0.28 μg/mL and a half-maximal effective concentration of 0.46 μg/mL.
中文翻译:

作为潜在杀菌剂和杀菌剂的新型 5-磺酰基-1,3,4-噻二唑取代黄酮类化合物:设计、合成、三维定量构效关系研究
黄酮类化合物是普遍存在的天然产物,由于其结构多样性、广谱药理活性和优异的环境相容性,为药物发现提供了来源。为了开发具有新颖作用机制和创新结构的抗菌和抗真菌药物,设计并合成了一系列新型5-磺酰基-1,3,4-噻二唑取代的黄酮类化合物,并评估了它们对七种农业常见植物病原微生物的生物活性。抗菌生物测定结果表明,大部分目标化合物对米黄单胞菌、立枯丝核菌和炭疽病菌均表现出良好的抑制作用。化合物1、3、7、9、13和14对米叶枯病菌表现出显着的抗菌活性。稻曲霉的 EC 50值低于 10 μg/mL,优于双甲噻唑 (70.89 μg/mL)。化合物2 (EC 50 = 0.41 μg/mL)在体内对立枯丝核菌表现出最有效的抑制效力,其保护作用与阳性对照多菌灵相当。初步机理研究表明,化合物2诱导菌丝无序缠结、菌丝表面收缩、细胞内容物外渗以及液泡膨胀和破裂,从而扰乱了正常菌丝生长。随后,基于可靠的三维定量构效关系(3D-QSAR)模型,设计并合成了具有良好抗真菌活性的化合物35-53 。 化合物49对立枯丝核菌表现出高效且优异的抗真菌活性,EC 50值为0.28 μg/mL,半数有效浓度为0.46 μg/mL。
更新日期:2024-03-14
中文翻译:

作为潜在杀菌剂和杀菌剂的新型 5-磺酰基-1,3,4-噻二唑取代黄酮类化合物:设计、合成、三维定量构效关系研究
黄酮类化合物是普遍存在的天然产物,由于其结构多样性、广谱药理活性和优异的环境相容性,为药物发现提供了来源。为了开发具有新颖作用机制和创新结构的抗菌和抗真菌药物,设计并合成了一系列新型5-磺酰基-1,3,4-噻二唑取代的黄酮类化合物,并评估了它们对七种农业常见植物病原微生物的生物活性。抗菌生物测定结果表明,大部分目标化合物对米黄单胞菌、立枯丝核菌和炭疽病菌均表现出良好的抑制作用。化合物1、3、7、9、13和14对米叶枯病菌表现出显着的抗菌活性。稻曲霉的 EC 50值低于 10 μg/mL,优于双甲噻唑 (70.89 μg/mL)。化合物2 (EC 50 = 0.41 μg/mL)在体内对立枯丝核菌表现出最有效的抑制效力,其保护作用与阳性对照多菌灵相当。初步机理研究表明,化合物2诱导菌丝无序缠结、菌丝表面收缩、细胞内容物外渗以及液泡膨胀和破裂,从而扰乱了正常菌丝生长。随后,基于可靠的三维定量构效关系(3D-QSAR)模型,设计并合成了具有良好抗真菌活性的化合物35-53 。 化合物49对立枯丝核菌表现出高效且优异的抗真菌活性,EC 50值为0.28 μg/mL,半数有效浓度为0.46 μg/mL。






























 京公网安备 11010802027423号
京公网安备 11010802027423号