当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Continuous Flow Synthesis of Benzotriazin-4(3H)-ones via Visible Light Mediated Nitrogen-Centered Norrish Reaction
Organic Letters ( IF 4.9 ) Pub Date : 2024-03-11 , DOI: 10.1021/acs.orglett.4c00248 Jorge García-Lacuna 1 , Marcus Baumann 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-03-11 , DOI: 10.1021/acs.orglett.4c00248 Jorge García-Lacuna 1 , Marcus Baumann 1
Affiliation
|
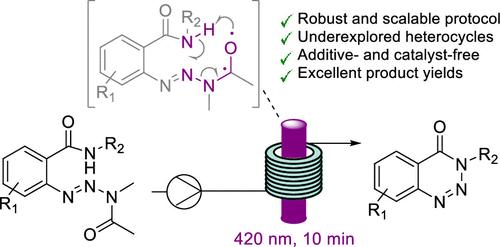
We report a new protocol for the synthesis of substituted benzotriazin-4(3H)-ones which are underrepresented heterocyclic scaffolds with important pharmacological properties. Our method exploits acyclic aryl triazine precursors that undergo a photocyclization reaction upon exposure to violet light (420 nm). Continuous flow reactor technology is exploited to afford excellent yields in only 10 min residence time with no additives or photocatalysts needed. The underlying reaction mechanism appears to be based on an unprecedented variation of the classical Norrish type II reaction with concomitant fragmentation and formation of N–N bonds. Scalability, process robustness, and green credentials of this intriguing transformation are highlighted.
中文翻译:

可见光介导氮中心诺里什反应连续流动合成苯并三嗪-4(3H)-酮
我们报告了一种合成取代苯并三嗪-4(3 H )-酮的新方案,该酮是具有重要药理学特性的代表性不足的杂环支架。我们的方法利用无环芳基三嗪前体,在暴露于紫光(420 nm)时发生光环化反应。利用连续流反应器技术,只需 10 分钟的停留时间即可获得优异的产率,且无需任何添加剂或光催化剂。潜在的反应机制似乎是基于经典 Norrish II 型反应的前所未有的变化,并伴随着断裂和 N-N 键的形成。这一有趣的转变的可扩展性、流程稳健性和绿色证书得到了强调。
更新日期:2024-03-11
中文翻译:

可见光介导氮中心诺里什反应连续流动合成苯并三嗪-4(3H)-酮
我们报告了一种合成取代苯并三嗪-4(3 H )-酮的新方案,该酮是具有重要药理学特性的代表性不足的杂环支架。我们的方法利用无环芳基三嗪前体,在暴露于紫光(420 nm)时发生光环化反应。利用连续流反应器技术,只需 10 分钟的停留时间即可获得优异的产率,且无需任何添加剂或光催化剂。潜在的反应机制似乎是基于经典 Norrish II 型反应的前所未有的变化,并伴随着断裂和 N-N 键的形成。这一有趣的转变的可扩展性、流程稳健性和绿色证书得到了强调。






























 京公网安备 11010802027423号
京公网安备 11010802027423号