当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A pyridinium-based strategy for lysine-selective protein modification and chemoproteomic profiling in live cells
Chemical Science ( IF 7.6 ) Pub Date : 2024-03-13 , DOI: 10.1039/d3sc05766f Chuan Wan 1 , Dongyan Yang 2 , Chunli Song 3 , Mingchan Liang 3 , Yuhao An 3 , Chenshan Lian 3 , Chuan Dai 3 , Yuxin Ye 3 , Feng Yin 3 , Rui Wang 3 , Zigang Li 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2024-03-13 , DOI: 10.1039/d3sc05766f Chuan Wan 1 , Dongyan Yang 2 , Chunli Song 3 , Mingchan Liang 3 , Yuhao An 3 , Chenshan Lian 3 , Chuan Dai 3 , Yuxin Ye 3 , Feng Yin 3 , Rui Wang 3 , Zigang Li 3, 4
Affiliation
|
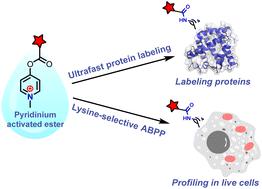
Protein active states are dynamically regulated by various modifications; thus, endogenous protein modification is an important tool for understanding protein functions and networks in complicated biological systems. Here we developed a new pyridinium-based approach to label lysine residues under physiological conditions that is low-toxicity, efficient, and lysine-selective. Furthermore, we performed a large-scale analysis of the ∼70% lysine-selective proteome in MCF-7 cells using activity-based protein profiling (ABPP). We quantifically assessed 1216 lysine-labeled peptides in cell lysates and identified 386 modified lysine sites including 43 mitochondrial-localized proteins in live MCF-7 cells. Labeled proteins significantly preferred the mitochondria. This pyridinium-based methodology demonstrates the importance of analyzing endogenous proteins under native conditions and provides a robust chemical strategy utilizing either lysine-selective protein labeling or spatiotemporal profiling in a living system.
中文翻译:

基于吡啶的活细胞赖氨酸选择性蛋白质修饰和化学蛋白质组学分析策略
蛋白质活性状态通过各种修饰动态调节;因此,内源蛋白质修饰是理解复杂生物系统中蛋白质功能和网络的重要工具。在这里,我们开发了一种基于吡啶的新方法,可以在生理条件下标记赖氨酸残基,该方法低毒、高效且具有赖氨酸选择性。此外,我们使用基于活性的蛋白质分析 (ABPP) 对 MCF-7 细胞中约 70% 的赖氨酸选择性蛋白质组进行了大规模分析。我们定量评估了细胞裂解物中的 1216 个赖氨酸标记肽,并鉴定了活 MCF-7 细胞中的 386 个修饰赖氨酸位点,包括 43 个线粒体定位蛋白。标记的蛋白质明显偏爱线粒体。这种基于吡啶鎓的方法证明了在天然条件下分析内源蛋白质的重要性,并提供了利用赖氨酸选择性蛋白质标记或生命系统中的时空分析的强大化学策略。
更新日期:2024-03-13
中文翻译:

基于吡啶的活细胞赖氨酸选择性蛋白质修饰和化学蛋白质组学分析策略
蛋白质活性状态通过各种修饰动态调节;因此,内源蛋白质修饰是理解复杂生物系统中蛋白质功能和网络的重要工具。在这里,我们开发了一种基于吡啶的新方法,可以在生理条件下标记赖氨酸残基,该方法低毒、高效且具有赖氨酸选择性。此外,我们使用基于活性的蛋白质分析 (ABPP) 对 MCF-7 细胞中约 70% 的赖氨酸选择性蛋白质组进行了大规模分析。我们定量评估了细胞裂解物中的 1216 个赖氨酸标记肽,并鉴定了活 MCF-7 细胞中的 386 个修饰赖氨酸位点,包括 43 个线粒体定位蛋白。标记的蛋白质明显偏爱线粒体。这种基于吡啶鎓的方法证明了在天然条件下分析内源蛋白质的重要性,并提供了利用赖氨酸选择性蛋白质标记或生命系统中的时空分析的强大化学策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号