当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sequence- and Structure-Specific tRNA Dihydrouridylation by hDUS2
ACS Central Science ( IF 12.7 ) Pub Date : 2024-03-12 , DOI: 10.1021/acscentsci.3c01382 Jingwei Ji 1 , Nathan J Yu 1 , Ralph E Kleiner 1
ACS Central Science ( IF 12.7 ) Pub Date : 2024-03-12 , DOI: 10.1021/acscentsci.3c01382 Jingwei Ji 1 , Nathan J Yu 1 , Ralph E Kleiner 1
Affiliation
|
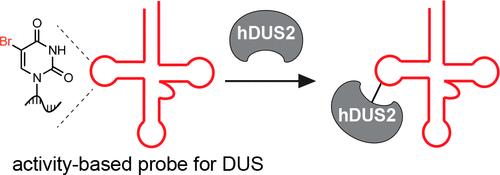
The post-transcriptional reduction of uridine to dihydrouridine (D) by dihydrouridine synthase (DUS) enzymes is among the most ubiquitous transformations in RNA biology. D is found at multiple sites in tRNAs, and studies in yeast have proposed that each of the four eukaryotic DUS enzymes modifies a different site; however, the molecular basis for this exquisite selectivity is unknown, and human DUS enzymes have remained largely uncharacterized. Here we investigate the substrate specificity of human dihydrouridine synthase 2 (hDUS2) using mechanism-based cross-linking with 5-bromouridine (5-BrUrd)-modified oligonucleotide probes and in vitro dihydrouridylation assays. We find that hDUS2 exclusively modifies U20 across diverse tRNA substrates and identify a minimal GU sequence within the tRNA D loop that underlies selective substrate modification. Further, we use our mechanism-based platform to screen small molecule inhibitors of hDUS2, a potential anticancer target. Our work elucidates the principles of substrate modification by a conserved DUS and provides a general platform for studying RNA modifying enzymes with sequence-defined activity-based probes.
中文翻译:

hDUS2 进行序列和结构特异性 tRNA 二氢尿苷化
二氢尿苷合酶 (DUS) 将尿苷转录后还原为二氢尿苷 (D),是 RNA 生物学中最普遍的转化之一。 D 在 tRNA 的多个位点上被发现,并且在酵母中的研究表明,四种真核 DUS 酶中的每一种都会修饰不同的位点;然而,这种精细选择性的分子基础尚不清楚,并且人类 DUS 酶在很大程度上仍未得到表征。在这里,我们使用基于机制的与 5-溴尿苷 (5-BrUrd) 修饰寡核苷酸探针的交联和体外二氢尿苷化测定来研究人二氢尿苷合酶 2 (hDUS2) 的底物特异性。我们发现 hDUS2 专门修饰跨不同 tRNA 底物的 U20,并在 tRNA D 环内识别出作为选择性底物修饰基础的最小 GU 序列。此外,我们使用基于机制的平台来筛选 hDUS2(一种潜在的抗癌靶标)的小分子抑制剂。我们的工作阐明了保守 DUS 进行底物修饰的原理,并为使用基于序列定义的活性的探针研究 RNA 修饰酶提供了一个通用平台。
更新日期:2024-03-12
中文翻译:

hDUS2 进行序列和结构特异性 tRNA 二氢尿苷化
二氢尿苷合酶 (DUS) 将尿苷转录后还原为二氢尿苷 (D),是 RNA 生物学中最普遍的转化之一。 D 在 tRNA 的多个位点上被发现,并且在酵母中的研究表明,四种真核 DUS 酶中的每一种都会修饰不同的位点;然而,这种精细选择性的分子基础尚不清楚,并且人类 DUS 酶在很大程度上仍未得到表征。在这里,我们使用基于机制的与 5-溴尿苷 (5-BrUrd) 修饰寡核苷酸探针的交联和体外二氢尿苷化测定来研究人二氢尿苷合酶 2 (hDUS2) 的底物特异性。我们发现 hDUS2 专门修饰跨不同 tRNA 底物的 U20,并在 tRNA D 环内识别出作为选择性底物修饰基础的最小 GU 序列。此外,我们使用基于机制的平台来筛选 hDUS2(一种潜在的抗癌靶标)的小分子抑制剂。我们的工作阐明了保守 DUS 进行底物修饰的原理,并为使用基于序列定义的活性的探针研究 RNA 修饰酶提供了一个通用平台。































 京公网安备 11010802027423号
京公网安备 11010802027423号