当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper/Chiral Phosphoric-Acid-Catalyzed Intramolecular Reductive Isocyanide-Alkene (1 + 2) Cycloaddition: Enantioselective Construction of 2-Azabicyclo[3.1.0]hexanes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-12 , DOI: 10.1021/jacs.4c00989 Sidi Cheng 1, 2 , Ting Yu 1, 2 , Jing Li 1, 2 , Yingxiang Liang 1, 2 , Shuang Luo 1, 2 , Qiang Zhu 1, 2, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-12 , DOI: 10.1021/jacs.4c00989 Sidi Cheng 1, 2 , Ting Yu 1, 2 , Jing Li 1, 2 , Yingxiang Liang 1, 2 , Shuang Luo 1, 2 , Qiang Zhu 1, 2, 3
Affiliation
|
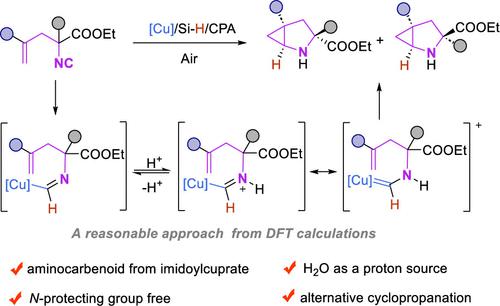
Enantioenriched 2-azabicyclo[3.1.0]hexanes are accessed from readily available allyl substituted α-isocyanoesters by intramolecular (1 + 2) cycloaddition with the olefinic moiety and isocyano carbon as the respective C2 and C1 units. Cyclopropanation is initiated by 1,1-hydrocupration of isocyanide followed by formimidoylcopper to copper α-aminocarbenoid equilibration and subsequent (1 + 2) cycloaddition. The unprecedented copper/chiral phosphoric acid (CPA) catalytic system can be operated in the presence of water under air, delivering a variety of 2-azabicyclo[3.1.0]hexanes containing an angular all-carbon quaternary stereocenter in good to excellent yields and enantioselectivity.
中文翻译:

铜/手性磷酸催化的分子内还原异氰化物-烯烃 (1 + 2) 环加成:2-氮杂双环[3.1.0]己烷的对映选择性构建
对映体富集的2-氮杂双环[3.1.0]己烷是通过烯基部分和异氰基碳作为各自的C2和C1单元的分子内(1+2)环加成从容易获得的烯丙基取代的α-异氰酸酯获得的。环丙烷化反应由异氰化物的 1,1-氢化反应引发,随后甲亚胺酰铜与铜 α-氨基类胡萝卜素平衡,随后进行 (1 + 2) 环加成。前所未有的铜/手性磷酸(CPA)催化系统可以在空气中有水的情况下运行,以良好至优异的收率提供各种含有角全碳四元立构中心的2-氮杂双环[3.1.0]己烷。对映选择性。
更新日期:2024-03-12
中文翻译:

铜/手性磷酸催化的分子内还原异氰化物-烯烃 (1 + 2) 环加成:2-氮杂双环[3.1.0]己烷的对映选择性构建
对映体富集的2-氮杂双环[3.1.0]己烷是通过烯基部分和异氰基碳作为各自的C2和C1单元的分子内(1+2)环加成从容易获得的烯丙基取代的α-异氰酸酯获得的。环丙烷化反应由异氰化物的 1,1-氢化反应引发,随后甲亚胺酰铜与铜 α-氨基类胡萝卜素平衡,随后进行 (1 + 2) 环加成。前所未有的铜/手性磷酸(CPA)催化系统可以在空气中有水的情况下运行,以良好至优异的收率提供各种含有角全碳四元立构中心的2-氮杂双环[3.1.0]己烷。对映选择性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号