当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
4-Fluorobenzyl cyanide, a sterically-hindered solvent expediting interfacial kinetics in lithium-ion batteries
Chemical Science ( IF 7.6 ) Pub Date : 2024-03-12 , DOI: 10.1039/d4sc00013g Mingsheng Qin 1, 2 , Ziqi Zeng 1 , Qiang Wu 1 , Xiaowei Liu 3 , Qijun Liu 1, 2 , Shijie Cheng 1 , Jia Xie 1
Chemical Science ( IF 7.6 ) Pub Date : 2024-03-12 , DOI: 10.1039/d4sc00013g Mingsheng Qin 1, 2 , Ziqi Zeng 1 , Qiang Wu 1 , Xiaowei Liu 3 , Qijun Liu 1, 2 , Shijie Cheng 1 , Jia Xie 1
Affiliation
|
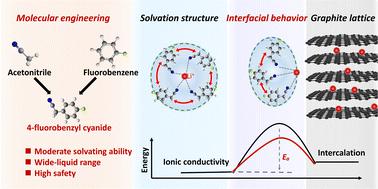
The electrochemical performance of lithium-ion batteries (LIBs) is plagued by sluggish interfacial kinetics. Fortunately, the Li+ solvation structure bridges the bulk electrolyte and interfacial chemistry, providing a pathway for promoting electrochemical kinetics in LIBs. Herein, we improve the interfacial kinetics by tuning the Li+ coordination chemistry based on solvent molecular engineering. Specifically, 4-fluorobenzyl cyanide (FBCN), featuring steric hindrance and a weak Lewis basic center, is designed to construct a bulky coordination structure with Li+, weakening ion–dipole interaction (Li+–solvents) but promoting coulombic attraction (Li+–anions) at a normal Li salt concentration. This sterically-controlled solvation chemistry reduces the interfacial barrier and thus contributes to improved rate performance, as demonstrated practically in LiFePO4//graphite pouch cells. This study provides fresh insights into solvent steric control and coordination chemistry engineering, opening a new avenue for enhancing electrochemical kinetics in LIBs.
中文翻译:

4-氟苄基氰,一种空间位阻溶剂,可促进锂离子电池中的界面动力学
锂离子电池(LIB)的电化学性能受到缓慢的界面动力学的困扰。幸运的是,Li +溶剂化结构连接了本体电解质和界面化学,为促进LIB中的电化学动力学提供了途径。在此,我们通过基于溶剂分子工程调整Li +配位化学来改善界面动力学。具体而言,4-氟苯甲基氰化物(FBCN)具有空间位阻和弱路易斯碱性中心,旨在与Li +构建庞大的配位结构,削弱离子偶极相互作用(Li + -溶剂),但促进库仑吸引力(Li + –阴离子)在正常的锂盐浓度下。这种空间控制的溶剂化化学降低了界面势垒,从而有助于提高倍率性能,正如 LiFePO 4 //石墨软包电池中的实际情况所证明的那样。这项研究为溶剂空间控制和配位化学工程提供了新的见解,为增强锂离子电池的电化学动力学开辟了新途径。
更新日期:2024-03-12
中文翻译:

4-氟苄基氰,一种空间位阻溶剂,可促进锂离子电池中的界面动力学
锂离子电池(LIB)的电化学性能受到缓慢的界面动力学的困扰。幸运的是,Li +溶剂化结构连接了本体电解质和界面化学,为促进LIB中的电化学动力学提供了途径。在此,我们通过基于溶剂分子工程调整Li +配位化学来改善界面动力学。具体而言,4-氟苯甲基氰化物(FBCN)具有空间位阻和弱路易斯碱性中心,旨在与Li +构建庞大的配位结构,削弱离子偶极相互作用(Li + -溶剂),但促进库仑吸引力(Li + –阴离子)在正常的锂盐浓度下。这种空间控制的溶剂化化学降低了界面势垒,从而有助于提高倍率性能,正如 LiFePO 4 //石墨软包电池中的实际情况所证明的那样。这项研究为溶剂空间控制和配位化学工程提供了新的见解,为增强锂离子电池的电化学动力学开辟了新途径。






























 京公网安备 11010802027423号
京公网安备 11010802027423号