当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights into the 5,5′-bis(1H-tetrazolyl)amine monohydrate (BTA·H2O) pyrolysis mechanism: integrated experimental and kinetic model analysis
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2024-03-12 , DOI: 10.1039/d3nj05438a Jianwei Zhang 1, 2 , Ling Chen 1, 2 , Peichen Han 1, 2 , Chunzhi Li 3 , Ye Yuan 3 , Bo Wu 4 , Feiyun Chen 1, 2 , Weidong He 1, 2
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2024-03-12 , DOI: 10.1039/d3nj05438a Jianwei Zhang 1, 2 , Ling Chen 1, 2 , Peichen Han 1, 2 , Chunzhi Li 3 , Ye Yuan 3 , Bo Wu 4 , Feiyun Chen 1, 2 , Weidong He 1, 2
Affiliation
|
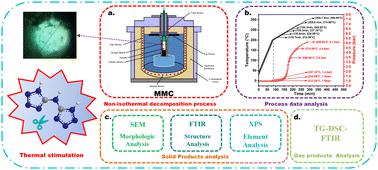
Nitrogen heterocycles as nitrogen-rich energetic materials have been widely popularized in energetic materials, especially in gun propellant application areas. Recently, bis-tetrazole compounds have attracted increasing focus for their various outstanding features, such as a high nitrogen content, insensitivity, and high thermal stability. In order to gain a greater in-depth understanding of the thermal decomposition behavior of such materials, the thermal decomposition process of BTA·H2O was systematically studied through a series of analyses. The thermal decomposition process and gas products were analyzed using TG-DSC-FTIR analysis. The results revealed that the pyrolysis process of BTA·H2O was a complex reaction, including three main mass loss stages. After the removal of crystal water in the BTA·H2O molecule, the thermal decomposition mechanism of BTA also was investigated by condensed-phase thermolysis by multiple mode calorimetry (MMC) assisted by SEM, FTIR, and XPS. The results revealed that the pyrolysis process of BTA was most likely to begin with a ring-opening reaction of the tetrazole ring, and the main gaseous products, including HCN, HN3, and NH3, were then released from the decomposition of BTA. According to the condensed-phase kinetics, a kinetic model describing the thermal decomposition process of BTA was proposed. Based on the above results and the force constant of the chemical bonds in BTA, a detailed mechanism for the thermal decomposition of BTA was proposed. In addition, thermal hazard prediction was performed for BTA. When the value of φ decreased from 64.23 to 47.65, TD24 decreased from 193.0 °C to 187.2 °C and TD8 decreased from 197.1 °C to 192.4 °C.
中文翻译:

深入了解5,5′-双(1H-四唑基)胺一水合物(BTA·H2O)热解机理:综合实验和动力学模型分析
氮杂环作为富氮含能材料,在含能材料特别是火炮发射药应用领域得到了广泛的推广。近年来,双四唑化合物因其各种优异的特性(例如高氮含量、不敏感性和高热稳定性)而引起了越来越多的关注。为了更深入地了解此类材料的热分解行为,通过一系列分析对BTA·H 2 O的热分解过程进行了系统研究。使用TG-DSC-FTIR分析热分解过程和气体产物。结果表明,BTA·H 2 O的热解过程是一个复杂的反应,包括三个主要的质量损失阶段。去除BTA·H 2 O分子中的结晶水后,通过多模量热法(MMC)凝聚相热解,并结合SEM、FTIR和XPS研究BTA的热分解机理。结果表明,BTA的热解过程最有可能以四唑环的开环反应开始,然后BTA分解释放出主要气态产物HCN、HN 3和NH 3 。根据凝聚相动力学,提出了描述BTA热分解过程的动力学模型。基于上述结果和BTA中化学键的力常数,提出了BTA热分解的详细机理。此外,还对 BTA 进行了热危险预测。当φ值从64.23下降到47.65时,TD24从193.0℃下降到187.2℃,TD8从197.1℃下降到192.4℃。
更新日期:2024-03-12
中文翻译:

深入了解5,5′-双(1H-四唑基)胺一水合物(BTA·H2O)热解机理:综合实验和动力学模型分析
氮杂环作为富氮含能材料,在含能材料特别是火炮发射药应用领域得到了广泛的推广。近年来,双四唑化合物因其各种优异的特性(例如高氮含量、不敏感性和高热稳定性)而引起了越来越多的关注。为了更深入地了解此类材料的热分解行为,通过一系列分析对BTA·H 2 O的热分解过程进行了系统研究。使用TG-DSC-FTIR分析热分解过程和气体产物。结果表明,BTA·H 2 O的热解过程是一个复杂的反应,包括三个主要的质量损失阶段。去除BTA·H 2 O分子中的结晶水后,通过多模量热法(MMC)凝聚相热解,并结合SEM、FTIR和XPS研究BTA的热分解机理。结果表明,BTA的热解过程最有可能以四唑环的开环反应开始,然后BTA分解释放出主要气态产物HCN、HN 3和NH 3 。根据凝聚相动力学,提出了描述BTA热分解过程的动力学模型。基于上述结果和BTA中化学键的力常数,提出了BTA热分解的详细机理。此外,还对 BTA 进行了热危险预测。当φ值从64.23下降到47.65时,TD24从193.0℃下降到187.2℃,TD8从197.1℃下降到192.4℃。







































 京公网安备 11010802027423号
京公网安备 11010802027423号