当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iridium-Catalyzed Asymmetric Hydrogenation of Heteroaromatics with Multiple N Atoms via Substrate Activation: An Entry to 4,5,6,7-Tetrahydropyrazolo[1,5-a]pyrimidine-3-carbonitrile Core of a Potent BTK Inhibitor
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-03-11 , DOI: 10.1021/acs.joc.3c02396
Mu-Wang Chen 1 , Hong-Wang Li 1 , Ying-Qi Wang 1 , Bo Wu 1 , Zheng Liu 1 , Xinzhong Lai 2 , Joerg Deerberg 2 , Yong-Gui Zhou 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-03-11 , DOI: 10.1021/acs.joc.3c02396
Mu-Wang Chen 1 , Hong-Wang Li 1 , Ying-Qi Wang 1 , Bo Wu 1 , Zheng Liu 1 , Xinzhong Lai 2 , Joerg Deerberg 2 , Yong-Gui Zhou 1
Affiliation
|
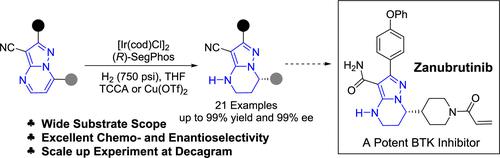
The chiral 4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidine is the key core skeleton of potent Bruton’s tyrosine kinase (BTK) inhibitor Zanubrutinib, and the catalyst-controlled asymmetric hydrogenation of planar multinuclear pyrimidine heteroarenes with multiple N atoms could provide an efficient route toward its synthesis. Owing to the strong aromaticity and poisoning effect toward chiral transition metal catalyst, asymmetric hydrogenation of pyrazolo[1,5-a]pyrimidines with multiple nitrogen atoms is still a challenge for synthesizing the chiral 4,5,6,7-tetrahydropyrazolo[1,5-a]-pyrimidine. Herein, an efficient iridium-catalyzed asymmetric hydrogenation of pyrazolo[1,5-a]pyrimidines has been developed using substrate activation strategy, with up to 99% ee. The decagram scale synthesis further demonstrated the potential and promise of this procedure in the synthesis of Zanubrutinib. In addition, a mechanistic study indicated that the hydrogenation starts with 1,2-hydrogenation.
中文翻译:

通过底物激活铱催化多 N 原子杂芳烃的不对称氢化:进入有效 BTK 抑制剂的 4,5,6,7-四氢吡唑并[1,5-a]嘧啶-3-甲腈核心
手性4,5,6,7-四氢吡唑并[1,5- a ]嘧啶是有效的布鲁顿酪氨酸激酶(BTK)抑制剂Zanubrutinib的关键核心骨架,以及催化剂控制的具有多个N的平面多核嘧啶杂芳烃的不对称氢化原子可以为其合成提供有效的途径。由于手性过渡金属催化剂的强芳香性和中毒效应,多氮原子吡唑并[1,5- a ]嘧啶的不对称氢化仍然是合成手性4,5,6,7-四氢吡唑并[1, 5- a ]-嘧啶。在此,我们利用底物活化策略开发了一种高效的铱催化吡唑并[1,5- a ]嘧啶不对称氢化反应,其ee高达99%。十克规模的合成进一步证明了该方法在 Zanubrutinib 合成中的潜力和前景。此外,机理研究表明氢化反应是从1,2-氢化反应开始的。
更新日期:2024-03-11
中文翻译:

通过底物激活铱催化多 N 原子杂芳烃的不对称氢化:进入有效 BTK 抑制剂的 4,5,6,7-四氢吡唑并[1,5-a]嘧啶-3-甲腈核心
手性4,5,6,7-四氢吡唑并[1,5- a ]嘧啶是有效的布鲁顿酪氨酸激酶(BTK)抑制剂Zanubrutinib的关键核心骨架,以及催化剂控制的具有多个N的平面多核嘧啶杂芳烃的不对称氢化原子可以为其合成提供有效的途径。由于手性过渡金属催化剂的强芳香性和中毒效应,多氮原子吡唑并[1,5- a ]嘧啶的不对称氢化仍然是合成手性4,5,6,7-四氢吡唑并[1, 5- a ]-嘧啶。在此,我们利用底物活化策略开发了一种高效的铱催化吡唑并[1,5- a ]嘧啶不对称氢化反应,其ee高达99%。十克规模的合成进一步证明了该方法在 Zanubrutinib 合成中的潜力和前景。此外,机理研究表明氢化反应是从1,2-氢化反应开始的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号