当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Catalytic Stability of Cs2O–P2O5 over Acid-Activated Montmorillonite for the Gas-Phase Dehydration of Monoethanolamine to Ethylenimine
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-03-11 , DOI: 10.1021/acs.iecr.3c04241 Gui-Qiu Huang 1, 2 , Dan Yang 1 , Chang Liu 1 , Han-Qing Ge 1 , Zhao-Tie Liu 1 , Zhong-Wen Liu 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-03-11 , DOI: 10.1021/acs.iecr.3c04241 Gui-Qiu Huang 1, 2 , Dan Yang 1 , Chang Liu 1 , Han-Qing Ge 1 , Zhao-Tie Liu 1 , Zhong-Wen Liu 1
Affiliation
|
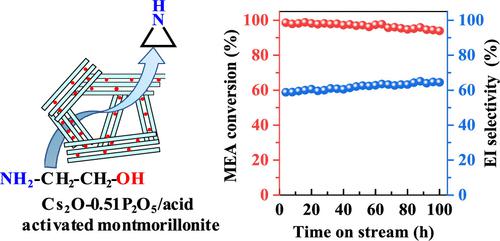
In this work, the gas-phase selective dehydration of monoethanolamine (MEA) to ethylenimine (EI) was studied by using cesium and phosphorus oxides (Cs2O–P2O5) over acid-activated montmorillonite (Acid-MMT) as a catalyst, and key factors affecting the catalytic stability are focused by operating the reaction for a time on stream (TOS) of 100 h. By keeping a fixed content of Cs2O over the catalyst, the change of the P2O5/Cs2O molar ratio from 0.16 to 0.58 leads to significantly varied MEA conversion and EI selectivity. Importantly, a stable EI yield of about 60% for a TOS of 100 h is achieved over the optimal catalyst, with a P2O5/Cs2O molar ratio of 0.51. Physicochemical properties of fresh/spent catalysts were characterized by powder X-ray diffraction, X-ray fluorescence, N2 adsorption–desorption isotherms, and temperature-programmed desorption of NH3 and CO2. Moreover, properties of the coke deposited over the spent catalysts were explored by thermal analysis and X-ray photoelectron spectroscopy. Results disclose that the acidic/basic sites play a key role for catalyzing the conversion of MEA to different products, and the catalyst with a higher amount of acidic/basic sites exhibits a lower deactivation rate. Although stronger acidic/basic sites are not directly involved in producing the targeted EI product, they promote the side reactions related to the coke deposition. The heteroatom-containing coke is beneficial to improving the EI selectivity via preferentially poisoning the stronger acidic/basic sites, which inhibits the unwanted side reactions. Considering the abundance of the precursors and the simple procedure of catalyst preparation, Cs2O–P2O5/Acid-MMT is a promising catalyst for the large-scale production of EI via gas-phase MEA dehydration.
中文翻译:

了解 Cs2O–P2O5 在酸活化蒙脱土上对单乙醇胺气相脱水生成乙烯亚胺的催化稳定性
在这项工作中,通过使用铯和磷的氧化物(Cs 2 O–P 2 O 5 )在酸活化蒙脱土(Acid-MMT)上作为催化剂,研究了单乙醇胺(MEA)气相选择性脱水生成乙烯亚胺(EI )的过程。催化剂,并通过运行反应时间(TOS)100小时来关注影响催化稳定性的关键因素。通过保持催化剂上Cs 2 O的固定含量,P 2 O 5 /Cs 2 O摩尔比从0.16变化到0.58导致MEA转化率和EI选择性显着变化。重要的是,在P 2 O 5 /Cs 2 O摩尔比为0.51的最佳催化剂上,TOS为100小时时,获得了约60%的稳定EI产率。通过粉末X射线衍射、X射线荧光、N 2吸附-解吸等温线以及NH 3和CO 2的程序升温解吸来表征新鲜/废催化剂的物理化学性质。此外,通过热分析和 X 射线光电子能谱探索了废催化剂上沉积的焦炭的性质。结果表明,酸/碱位点对于催化MEA转化为不同的产物起着关键作用,酸/碱位点数量较多的催化剂表现出较低的失活率。虽然较强的酸性/碱性位点不直接参与目标 EI 产品的生产,但它们会促进与焦炭沉积相关的副反应。含杂原子的焦炭通过优先毒害较强的酸性/碱性位点,抑制不需要的副反应,有利于提高 EI 选择性。考虑到前驱体的丰富性和催化剂制备过程的简单性,Cs 2 O–P 2 O 5 /Acid-MMT是一种很有前景的通过气相MEA脱水大规模生产EI的催化剂。
更新日期:2024-03-11
中文翻译:

了解 Cs2O–P2O5 在酸活化蒙脱土上对单乙醇胺气相脱水生成乙烯亚胺的催化稳定性
在这项工作中,通过使用铯和磷的氧化物(Cs 2 O–P 2 O 5 )在酸活化蒙脱土(Acid-MMT)上作为催化剂,研究了单乙醇胺(MEA)气相选择性脱水生成乙烯亚胺(EI )的过程。催化剂,并通过运行反应时间(TOS)100小时来关注影响催化稳定性的关键因素。通过保持催化剂上Cs 2 O的固定含量,P 2 O 5 /Cs 2 O摩尔比从0.16变化到0.58导致MEA转化率和EI选择性显着变化。重要的是,在P 2 O 5 /Cs 2 O摩尔比为0.51的最佳催化剂上,TOS为100小时时,获得了约60%的稳定EI产率。通过粉末X射线衍射、X射线荧光、N 2吸附-解吸等温线以及NH 3和CO 2的程序升温解吸来表征新鲜/废催化剂的物理化学性质。此外,通过热分析和 X 射线光电子能谱探索了废催化剂上沉积的焦炭的性质。结果表明,酸/碱位点对于催化MEA转化为不同的产物起着关键作用,酸/碱位点数量较多的催化剂表现出较低的失活率。虽然较强的酸性/碱性位点不直接参与目标 EI 产品的生产,但它们会促进与焦炭沉积相关的副反应。含杂原子的焦炭通过优先毒害较强的酸性/碱性位点,抑制不需要的副反应,有利于提高 EI 选择性。考虑到前驱体的丰富性和催化剂制备过程的简单性,Cs 2 O–P 2 O 5 /Acid-MMT是一种很有前景的通过气相MEA脱水大规模生产EI的催化剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号