当前位置:
X-MOL 学术
›
Toxicol. Appl. Pharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Predicting the in vivo developmental toxicity of fenarimol from in vitro toxicity data using PBTK modelling-facilitated reverse dosimetry approach
Toxicology and Applied Pharmacology ( IF 3.3 ) Pub Date : 2024-03-01 , DOI: 10.1016/j.taap.2024.116879 Manisha Bhateria 1 , Isha Taneja 2 , Kajal Karsauliya 1 , Ashish Kumar Sonker 3 , Yukihiro Shibata 4 , Hiromi Sato 4 , Sheelendra Pratap Singh 3 , Akihiro Hisaka 4
Toxicology and Applied Pharmacology ( IF 3.3 ) Pub Date : 2024-03-01 , DOI: 10.1016/j.taap.2024.116879 Manisha Bhateria 1 , Isha Taneja 2 , Kajal Karsauliya 1 , Ashish Kumar Sonker 3 , Yukihiro Shibata 4 , Hiromi Sato 4 , Sheelendra Pratap Singh 3 , Akihiro Hisaka 4
Affiliation
|
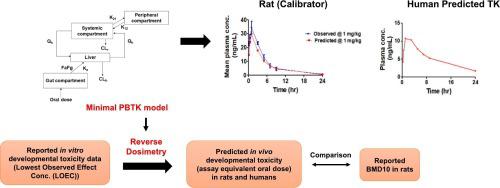
methods are widely used in modern toxicological testing; however, the data cannot be directly employed for risk assessment. toxicity of chemicals can be predicted from data using physiologically based toxicokinetic (PBTK) modelling-facilitated reverse dosimetry (PBTK-RD). In this study, a minimal-PBTK model was constructed to predict the kinetic profile of fenarimol (FNL) in rats and humans. The model was verified by comparing the observed and predicted pharmacokinetics of FNL for rats (calibrator) and further applied to humans. Using the PBTK-RD approach, the reported developmental toxicity data for FNL was translated to dose-response data to predict the assay equivalent oral dose in rats and humans. The predicted assay equivalent rat oral dose (36.46 mg/kg) was comparable to the literature reported BMD10 value (22.8 mg/kg). The model was also employed to derive the chemical-specific adjustment factor (CSAF) for interspecies toxicokinetics variability of FNL. Further, Monte Carlo simulations were performed to predict the population variability in the plasma concentration of FNL and to derive CSAF for intersubject human kinetic differences. The comparison of CSAF values for interspecies and intersubject toxicokinetic variability with their respective default values revealed that the applied uncertainty factors were adequately protective.
中文翻译:

使用 PBTK 建模辅助的反向剂量测定方法根据体外毒性数据预测芬那莫的体内发育毒性
现代毒理学检测中广泛使用的方法;然而,这些数据不能直接用于风险评估。化学品的毒性可以使用基于生理的毒代动力学 (PBTK) 建模辅助反向剂量测定 (PBTK-RD) 的数据进行预测。在本研究中,构建了最小 PBTK 模型来预测芬那莫尔 (FNL) 在大鼠和人类中的动力学特征。通过比较大鼠(校准品)观察到的和预测的 FNL 药代动力学,对该模型进行了验证,并进一步应用于人类。使用 PBTK-RD 方法,将报告的 FNL 发育毒性数据转化为剂量反应数据,以预测大鼠和人类的测定等效口服剂量。预测的大鼠口服等效剂量(36.46 mg/kg)与文献报道的 BMD10 值(22.8 mg/kg)相当。该模型还用于推导 FNL 种间毒代动力学变异的化学特异性调整因子 (CSAF)。此外,进行蒙特卡罗模拟以预测 FNL 血浆浓度的群体变异性,并推导出受试者间人类动力学差异的 CSAF。将种间和受试者间毒代动力学变异性的 CSAF 值与其各自的默认值进行比较表明,所应用的不确定性因素具有充分的保护作用。
更新日期:2024-03-01
中文翻译:

使用 PBTK 建模辅助的反向剂量测定方法根据体外毒性数据预测芬那莫的体内发育毒性
现代毒理学检测中广泛使用的方法;然而,这些数据不能直接用于风险评估。化学品的毒性可以使用基于生理的毒代动力学 (PBTK) 建模辅助反向剂量测定 (PBTK-RD) 的数据进行预测。在本研究中,构建了最小 PBTK 模型来预测芬那莫尔 (FNL) 在大鼠和人类中的动力学特征。通过比较大鼠(校准品)观察到的和预测的 FNL 药代动力学,对该模型进行了验证,并进一步应用于人类。使用 PBTK-RD 方法,将报告的 FNL 发育毒性数据转化为剂量反应数据,以预测大鼠和人类的测定等效口服剂量。预测的大鼠口服等效剂量(36.46 mg/kg)与文献报道的 BMD10 值(22.8 mg/kg)相当。该模型还用于推导 FNL 种间毒代动力学变异的化学特异性调整因子 (CSAF)。此外,进行蒙特卡罗模拟以预测 FNL 血浆浓度的群体变异性,并推导出受试者间人类动力学差异的 CSAF。将种间和受试者间毒代动力学变异性的 CSAF 值与其各自的默认值进行比较表明,所应用的不确定性因素具有充分的保护作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号