Topics in Current Chemistry ( IF 7.1 ) Pub Date : 2024-03-08 , DOI: 10.1007/s41061-024-00456-x Biquan Xiong 1 , Minjing Yuan 1 , Chonghao Shi 1 , Longzhi Zhu 1 , Fan Cao 1 , Weifeng Xu 1 , Yining Ren 1 , Yu Liu 1 , Ke-Wen Tang 1
|
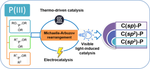
Organophosphorus compounds have long been considered valuable in both organic synthesis and life science. P(III)-nucleophiles, such as phosphites, phosphonites, and diaryl/alkyl phosphines, are particularly noteworthy as phosphorylation reagents for their ability to form new P−C bonds, producing more stable, ecofriendly, and cost-effective organophosphorus compounds. These nucleophiles follow similar phosphorylation routes as in the functionalization of P−H bonds and P−OH bonds. Activation can occur through photocatalytic, electrocatalytic, or thermo-driven reactions, often in coordination with a Michaelis–Arbuzov-trpe rearrangement process, to produce the desired products. As such, this review offers a thorough overview of the phosphorylated transformation and potential mechanisms of P(III)-nucleophiles, specifically focusing on developments since 2010. Notably, this review may provide researchers with valuable insights into designing and synthesizing functionalized organophosphorus compounds from P(III)-nucleophiles, guiding future advancements in both research and practical applications.
Graphical Abstract
中文翻译:

应用 P(III)-亲核试剂通过 Michaelis-Arbuzov 型重排创建新 P−C 键的最新进展
有机磷化合物长期以来被认为在有机合成和生命科学中有价值。 P(III)-亲核试剂,例如亚磷酸酯、亚膦酸酯和二芳基/烷基膦,作为磷酸化试剂特别值得注意,因为它们能够形成新的 P−C 键,从而产生更稳定、环境友好且具有成本效益的有机磷化合物。这些亲核试剂遵循与 P−H 键和 P−OH 键功能化类似的磷酸化途径。活化可以通过光催化、电催化或热驱动反应发生,通常与 Michaelis-Arbuzov-trpe 重排过程配合,以产生所需的产物。因此,这篇综述对 P(III)-亲核试剂的磷酸化转化和潜在机制进行了全面概述,特别关注 2010 年以来的发展。值得注意的是,这篇综述可能为研究人员从 P(III)-亲核试剂设计和合成功能化有机磷化合物提供宝贵的见解。 (III)-亲核试剂,指导研究和实际应用的未来进展。































 京公网安备 11010802027423号
京公网安备 11010802027423号