Cardiovascular Toxicology ( IF 3.4 ) Pub Date : 2024-03-07 , DOI: 10.1007/s12012-024-09838-5 Jinhui Zhang 1 , Xili Zhang 2 , Xunqiang Liu 1 , Huanjun Chen 1 , Jifeng Wang 1 , Min Ji 1
|
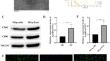
Abdominal aortic aneurysm (AAA) is a chronic vascular degenerative disease. Vascular smooth muscle cells (VSMCs) are essential for maintaining the integrity of healthy blood vessels. Macrophages play an important role in the inflammatory process of AAA. However, the effect of macrophage-derived exosome LncRNA PVT1 on VSMCs is unclear. Exosomes from M1 macrophages (M1φ-exos) were isolated and identified. The expression of LncRNA PVT1 in M1φ-exos was determined. AAA cell model was constructed by treating VSMCs with Ang-II. AAA cell model was treated with M1φ exosomes transfected with si-LncRNA PVT1 (M1φsi–LncRNA PVT1-exo). VSMCs were transfected with miR-186-5p mimic and oe-HMGB1. Cell viability was detected by CCK-8. The accumulation of LDH was detected by ELISA. Western blot was used to detect the expression of HMGB1, inflammatory factors (IL-6, TNF-α and IL-1β) and pyroptosis-related proteins (GSDMD, N-GSDMD, ASC, NLRP3, Caspase-1 and Cleaved-Capase-1). Cell pyroptosis rate was detected by flow cytometry. At the same time, the targeting relationship between miR-186-5p and LncRNA PVT1 and HMGB1 was verified by double fluorescein experiment. Exosomes from M1φ were successfully extracted. The expression of LncRNA PVT1 in M1φ-exos was significantly increased. M1φ-exo promotes inflammation and pyroptosis of VSMCs. M1φsi−LncRNA PVT1-exos inhibited the inflammation and pyroptosis of VSMCs. LncRNA PVT1 can sponge miR-186-5p mimic to regulate HMGB1 expression. MiR-186-5p mimic further inhibited inflammation and pyroptosis induced by M1φsi−LncRNA PVT1-exos. However, oe-HMGB1 could inhibit the reversal effect of miR-186-5p mimic. LncRNA PVT1 in exosomes secreted by M1φ can regulate HMGB1 by acting as ceRNA on sponge miR-186-5p, thereby promoting cell inflammatory and pyroptosis and accelerating AAA progression.
中文翻译:

M1巨噬细胞衍生的外泌体LncRNA PVT1通过抑制miR-186-5p和调节HMGB1促进腹主动脉瘤血管平滑肌细胞炎症和焦亡
腹主动脉瘤(AAA)是一种慢性血管退行性疾病。血管平滑肌细胞(VSMC)对于维持健康血管的完整性至关重要。巨噬细胞在 AAA 的炎症过程中发挥重要作用。然而,巨噬细胞来源的外泌体 LncRNA PVT1 对 VSMC 的影响尚不清楚。分离并鉴定了来自 M1 巨噬细胞的外泌体 (M1φ-exos)。测定了 M1φ-exos 中 LncRNA PVT1 的表达。 Ang-II处理VSMCs构建AAA细胞模型。用转染 si-LncRNA PVT1 的 M1φ 外泌体(M1φ si-LncRNA PVT1 -exo)处理 AAA 细胞模型。用 miR-186-5p 模拟物和 oe-HMGB1 转染 VSMC。通过CCK-8检测细胞活力。通过ELISA检测LDH的积累。 Western blot检测HMGB1、炎症因子(IL-6、TNF-α、IL-1β)及细胞焦亡相关蛋白(GSDMD、N-GSDMD、ASC、NLRP3、Caspase-1、Cleaved-Capase-)的表达情况。 1).流式细胞术检测细胞焦亡率。同时通过双荧光素实验验证了miR-186-5p与LncRNA PVT1和HMGB1之间的靶向关系。成功提取 M1φ 的外泌体。 M1φ-exos中LncRNA PVT1的表达显着增加。 M1φ-exo 促进 VSMC 炎症和焦亡。 M1φ si−LncRNA PVT1 -exos 抑制 VSMC 的炎症和焦亡。 LncRNA PVT1 可以海绵 miR-186-5p 模拟物来调节 HMGB1 表达。 MiR-186-5p 模拟物进一步抑制 M1φ si−LncRNA PVT1 -exos 诱导的炎症和细胞焦亡。然而,oe-HMGB1可以抑制miR-186-5p模拟物的逆转作用。 M1φ分泌的外泌体中的LncRNA PVT1可以通过充当海绵miR-186-5p上的ceRNA来调节HMGB1,从而促进细胞炎症和细胞焦亡,加速AAA进展。































 京公网安备 11010802027423号
京公网安备 11010802027423号