当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Insights into the Photoinduced Dimeric Gold-Catalyzed Divergent Dechloroalkylation of gem-Dichloroalkanes with Alkenes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-06 , DOI: 10.1021/jacs.3c14352
Kaifeng Wang 1 , Xiaoguang Bao 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-06 , DOI: 10.1021/jacs.3c14352
Kaifeng Wang 1 , Xiaoguang Bao 1, 2
Affiliation
|
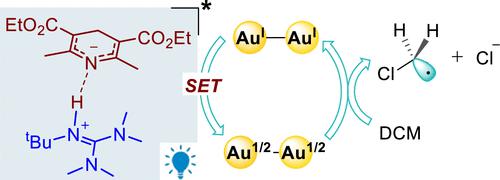
The employment of dinuclear Au(I) catalysts in photomediated modern organic transformations has attracted significant attention over the past decade, which commonly demonstrates unique catalytic performance compared with the corresponding mononuclear gold complexes. Nevertheless, detailed mechanisms of dinuclear gold catalysis remain ambiguous, and further mechanistic understanding is highly desirable. Herein, computational studies were carried out to gain mechanistic insights into the photoinduced dinuclear gold-catalyzed divergent dechloroalkylation of gem-dichloroalkanes. Computational results suggest that a proton transfer from the additive, Hantzsch ester (HE), to the base, guanidine, could lead to an ionic pair complex, which is ready to undergo excitation under blue light irradiation to result in the corresponding triplet excited state. Then, the excited complex might undergo oxidative quenching with the dinuclear gold photocatalyst [AuI–AuI]2+, via a single-electron-transfer (SET) step to afford an unusual [Au1/2–Au1/2]+ dinuclear species. The corresponding mononuclear gold catalyst, [AuI]+, however, is not ready to enable the analogous step to give a [Au0] species, which might account for the unique characteristics of dinuclear gold catalysis. Subsequently, the formed [Au1/2–Au1/2]+ intermediate could trigger a Cl-atom transfer from dichloromethane in an inner-sphere manner to furnish a critical chloromethyl radical. Next, the resulting chloromethyl radical could attack the alkenyl moiety of substrates to generate the corresponding alkyl radicals. Then, three possible mechanistic pathways were explored to rationalize the substrate-dependent divergent transformations in this protocol. The main factors responsible for the diversified transformations were discussed.
中文翻译:

宝石二氯烷烃与烯烃光诱导二聚体金催化发散脱氯烷基化反应的计算见解
在过去的十年中,双核Au(I)催化剂在光介导的现代有机转化中的应用引起了人们的广泛关注,与相应的单核金配合物相比,它通常表现出独特的催化性能。然而,双核金催化的详细机制仍然不明确,非常需要进一步的机制理解。在此,进行了计算研究,以获得对偕二氯烷烃的光诱导双核金催化发散脱氯烷基化的机理见解。计算结果表明,质子从添加剂 Hantzsch 酯 ( HE ) 转移到碱基胍,可能会形成离子对复合物,该复合物准备在蓝光照射下进行激发,从而产生相应的三重激发态。然后,激发的复合物可能会通过双核金光催化剂[Au I –Au I ] 2+进行氧化猝灭,通过单电子转移 (SET) 步骤提供不寻常的[Au 1/2 –Au 1/2 ] +双核物种。然而,相应的单核金催化剂[Au I ] +尚未准备好进行类似的步骤来产生[Au 0 ]物质,这可能解释了双核金催化的独特特征。 随后,形成的[Au 1/2 –Au 1/2 ] +中间体可以以内球方式触发二氯甲烷中的Cl原子转移,从而提供关键的氯甲基自由基。接下来,产生的氯甲基自由基可以攻击底物的烯基部分,生成相应的烷基自由基。然后,探索了三种可能的机制途径,以合理化该协议中依赖于底物的发散转化。讨论了多元化转型的主要因素。
更新日期:2024-03-06
中文翻译:

宝石二氯烷烃与烯烃光诱导二聚体金催化发散脱氯烷基化反应的计算见解
在过去的十年中,双核Au(I)催化剂在光介导的现代有机转化中的应用引起了人们的广泛关注,与相应的单核金配合物相比,它通常表现出独特的催化性能。然而,双核金催化的详细机制仍然不明确,非常需要进一步的机制理解。在此,进行了计算研究,以获得对偕二氯烷烃的光诱导双核金催化发散脱氯烷基化的机理见解。计算结果表明,质子从添加剂 Hantzsch 酯 ( HE ) 转移到碱基胍,可能会形成离子对复合物,该复合物准备在蓝光照射下进行激发,从而产生相应的三重激发态。然后,激发的复合物可能会通过双核金光催化剂[Au I –Au I ] 2+进行氧化猝灭,通过单电子转移 (SET) 步骤提供不寻常的[Au 1/2 –Au 1/2 ] +双核物种。然而,相应的单核金催化剂[Au I ] +尚未准备好进行类似的步骤来产生[Au 0 ]物质,这可能解释了双核金催化的独特特征。 随后,形成的[Au 1/2 –Au 1/2 ] +中间体可以以内球方式触发二氯甲烷中的Cl原子转移,从而提供关键的氯甲基自由基。接下来,产生的氯甲基自由基可以攻击底物的烯基部分,生成相应的烷基自由基。然后,探索了三种可能的机制途径,以合理化该协议中依赖于底物的发散转化。讨论了多元化转型的主要因素。

































 京公网安备 11010802027423号
京公网安备 11010802027423号