当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Alkyne Addition to Nitrones Enabled by Tunable Axially Chiral Imidazole-Based P,N-Ligands
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-06 , DOI: 10.1021/jacs.4c00873 Shengkang Yin 1 , Kendall N Weeks 1 , Aaron Aponick 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-06 , DOI: 10.1021/jacs.4c00873 Shengkang Yin 1 , Kendall N Weeks 1 , Aaron Aponick 1
Affiliation
|
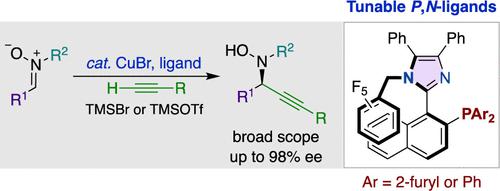
Although catalytic enantioselective alkyne addition is an established method for the synthesis of chiral propargylic alcohols and amines, addition to nitrones presents unique challenges, and no general chiral catalyst system has been developed. In this manuscript, we report the first Cu-catalyzed enantioselective alkyne addition to nitrones utilizing tunable axially chiral imidazole-based P,N-ligands. Our approach effectively overcomes difficulties in both reactivity and selectivity, resulting in a simple Cu-catalyzed protocol. The reaction accommodates a wide range of nitrones and alkynes, enabling the streamlined synthesis of chiral propargyl N-hydroxylamines via the enantioselective C–C bond formation. A diverse array of optically active nitrogen-containing compounds, including chiral hydroxylamines, can be accessed directly through facile transformations of the reaction products.
中文翻译:

可调节轴向手性咪唑基 P,N-配体实现催化对映选择性炔烃与硝酮加成
尽管催化对映选择性炔烃加成是合成手性炔丙醇和胺的既定方法,但硝酮的加成提出了独特的挑战,并且尚未开发出通用的手性催化剂体系。在这篇手稿中,我们报道了利用可调节的轴向手性咪唑基P 、 N配体,首次将铜催化的对映选择性炔烃加成到硝酮上。我们的方法有效地克服了反应性和选择性方面的困难,从而产生了简单的铜催化方案。该反应适用于多种硝酮和炔烃,能够通过对映选择性 C-C 键形成来简化手性炔丙基N-羟胺的合成。通过反应产物的简单转化,可以直接获得多种光学活性含氮化合物,包括手性羟胺。
更新日期:2024-03-06
中文翻译:

可调节轴向手性咪唑基 P,N-配体实现催化对映选择性炔烃与硝酮加成
尽管催化对映选择性炔烃加成是合成手性炔丙醇和胺的既定方法,但硝酮的加成提出了独特的挑战,并且尚未开发出通用的手性催化剂体系。在这篇手稿中,我们报道了利用可调节的轴向手性咪唑基P 、 N配体,首次将铜催化的对映选择性炔烃加成到硝酮上。我们的方法有效地克服了反应性和选择性方面的困难,从而产生了简单的铜催化方案。该反应适用于多种硝酮和炔烃,能够通过对映选择性 C-C 键形成来简化手性炔丙基N-羟胺的合成。通过反应产物的简单转化,可以直接获得多种光学活性含氮化合物,包括手性羟胺。































 京公网安备 11010802027423号
京公网安备 11010802027423号