当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Chemoinformatic Analysis of Fluorinated Piperidines as 3D Fragments for Fragment-Based Drug Discovery
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-03-07 , DOI: 10.1021/acs.joc.4c00143
Myriam Le Roch 1 , Jacques Renault 1 , Gilles Argouarch 1 , Elena Lenci 2 , Andrea Trabocchi 2 , Thierry Roisnel 3 , Nicolas Gouault 1 , Claudia Lalli 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-03-07 , DOI: 10.1021/acs.joc.4c00143
Myriam Le Roch 1 , Jacques Renault 1 , Gilles Argouarch 1 , Elena Lenci 2 , Andrea Trabocchi 2 , Thierry Roisnel 3 , Nicolas Gouault 1 , Claudia Lalli 1
Affiliation
|
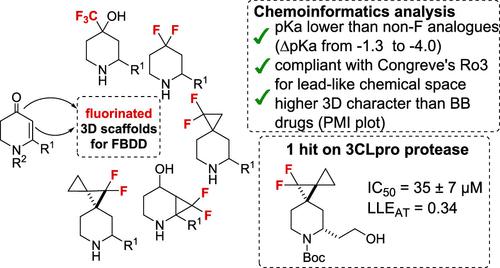
The concise synthesis of a small library of fluorinated piperidines from readily available dihydropyridinone derivatives has been described. The effect of the fluorination on different positions has then been evaluated by chemoinformatic tools. In particular, the compounds' pKa's have been calculated, revealing that the fluorine atoms notably lowered their basicity, which is correlated to the affinity for hERG channels resulting in cardiac toxicity. The “lead-likeness” and three-dimensionality have also been evaluated to assess their ability as useful fragments for drug design. A random screening on a panel of representative proteolytic enzymes was then carried out and revealed that one scaffold is recognized by the catalytic pocket of 3CLPro (main protease of SARS-CoV-2 coronavirus).
中文翻译:

用于基于片段的药物发现的 3D 片段氟化哌啶的合成和化学信息学分析
已经描述了从容易获得的二氢吡啶酮衍生物中简明合成小型氟化哌啶库。然后通过化学信息学工具评估氟化对不同位置的影响。特别是,计算了化合物的pKa值,表明氟原子显着降低了它们的碱性,这与导致心脏毒性的 hERG 通道的亲和力相关。还对“先导相似性”和三维度进行了评估,以评估它们作为药物设计有用片段的能力。然后对一组代表性蛋白水解酶进行随机筛选,结果发现一个支架被 3CL Pro (SARS-CoV-2 冠状病毒的主要蛋白酶)的催化口袋识别。
更新日期:2024-03-07
中文翻译:

用于基于片段的药物发现的 3D 片段氟化哌啶的合成和化学信息学分析
已经描述了从容易获得的二氢吡啶酮衍生物中简明合成小型氟化哌啶库。然后通过化学信息学工具评估氟化对不同位置的影响。特别是,计算了化合物的pKa值,表明氟原子显着降低了它们的碱性,这与导致心脏毒性的 hERG 通道的亲和力相关。还对“先导相似性”和三维度进行了评估,以评估它们作为药物设计有用片段的能力。然后对一组代表性蛋白水解酶进行随机筛选,结果发现一个支架被 3CL Pro (SARS-CoV-2 冠状病毒的主要蛋白酶)的催化口袋识别。

































 京公网安备 11010802027423号
京公网安备 11010802027423号