当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photochemical C(sp3)–H Activation for Diversity-Oriented Synthesis of 3-Functionalized Oxindoles
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-03-05 , DOI: 10.1021/acs.joc.3c02953
Hao Hou 1 , Wei Ou 1 , Chenliang Su 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-03-05 , DOI: 10.1021/acs.joc.3c02953
Hao Hou 1 , Wei Ou 1 , Chenliang Su 1
Affiliation
|
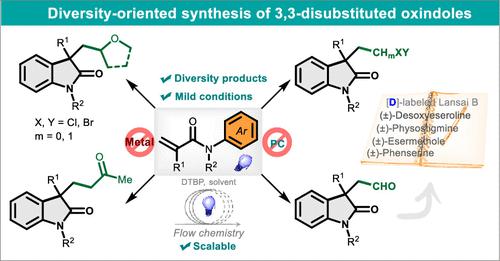
Heteroatom-adjacent C(sp3) radical cyclization of N-arylacrylamides provides a straightforward pathway to synthesize valuable 3-functionalized oxindoles. Traditional cyclization reactions normally require harsh conditions or transition-metal catalysts. Here, we developed a metal-free, diversity-oriented synthesis of 3-functionalized oxindoles via photochemically induced selective cleavage of C(sp3)–H bonds. A variety of 3-substituted oxindoles with functionalities such as ethers, polyhalogens, benzyl, and formyl groups can be obtained by a rational design. This strategy is characterized by its simple operation and mild conditions, aligning well with the developmental requirements for sustainable chemistry. The gram-scale continuous-flow synthesis and efficient construction of bioactive molecules highlight its practical utility.
中文翻译:

光化学 C(sp3)–H 活化用于 3-官能化羟吲哚的多样性合成
N-芳基丙烯酰胺的杂原子相邻C(sp 3 )自由基环化提供了合成有价值的3-官能化羟吲哚的直接途径。传统的环化反应通常需要苛刻的条件或过渡金属催化剂。在这里,我们通过光化学诱导 C(sp 3 )–H 键的选择性裂解,开发了一种无金属、多样性导向的 3 功能化羟吲哚合成方法。通过合理设计可以得到多种具有醚、多卤、苄基、甲酰基等官能团的3-取代羟吲哚。该策略操作简单、条件温和,符合可持续化学的发展要求。克级连续流动合成和生物活性分子的高效构建凸显了其实用性。
更新日期:2024-03-05
中文翻译:

光化学 C(sp3)–H 活化用于 3-官能化羟吲哚的多样性合成
N-芳基丙烯酰胺的杂原子相邻C(sp 3 )自由基环化提供了合成有价值的3-官能化羟吲哚的直接途径。传统的环化反应通常需要苛刻的条件或过渡金属催化剂。在这里,我们通过光化学诱导 C(sp 3 )–H 键的选择性裂解,开发了一种无金属、多样性导向的 3 功能化羟吲哚合成方法。通过合理设计可以得到多种具有醚、多卤、苄基、甲酰基等官能团的3-取代羟吲哚。该策略操作简单、条件温和,符合可持续化学的发展要求。克级连续流动合成和生物活性分子的高效构建凸显了其实用性。


































 京公网安备 11010802027423号
京公网安备 11010802027423号