当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
2,3-Dihydroquinazolin-4(1H)-ones and quinazolin-4(3H)-ones as broad-spectrum cytotoxic agents and their impact on tubulin polymerisation
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2024-03-06 , DOI: 10.1039/d3md00600j Nicholas S. O'Brien 1 , Jayne Gilbert 2 , Adam McCluskey 1 , Jennette A. Sakoff 2
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2024-03-06 , DOI: 10.1039/d3md00600j Nicholas S. O'Brien 1 , Jayne Gilbert 2 , Adam McCluskey 1 , Jennette A. Sakoff 2
Affiliation
|
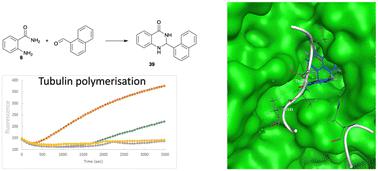
Tubulin plays a central role in mitosis and has been the target of multiple anticancer drugs, including paclitaxel. Herein two separate families of 2,3-dihydroquinazoline-4(1H)-ones and quinazoline-4(3H) ones, comprising 57 compounds in total, were synthesised. Screening against a broad panel of human cancer cell lines (HT29 colon, U87 and SJ-G2 glioblastoma, MCF-7 breast, A2780 ovarian, H460 lung, A431 skin, Du145 prostate, BE2-C neuroblastoma, and MIA pancreas) reveals these analogues to be broad spectrum cytotoxic compounds. Of particular note, 2-styrylquinazolin-4(3H)-one 51, 2-(4-hydroxystyryl)quinazolin-4(3H)-one 63, 2-(2-methoxystyryl)quinazolin-4(3H)-one 64 and 2-(3-methoxystyryl)quinazolin-4(3H)-one 65 and 2-(naphthalen-1-yl)-2,3-dihydroquinazolin-4(1H)-one 39 exhibited sub-μM potency growth inhibition values. Of these 1-naphthyl 39 has activity <50 nM against the HT29, U87, A2780, H460 and BE2-C cell lines. Molecular modelling of these compounds, e.g. 2-(naphthalen-1-yl)-2,3-dihydroquinazolin-4(1H)-one 39, 2-(2-methoxystyryl)quinazolin-4(3H)-one 64, 2-(3-methoxystyryl)quinazolin-4(3H)-one 65, and 2-(4-methoxystyryl)quinazolin-4(3H)-one 50 docked to the known tubulin polymerisation inhibitor sites highlighted well conserved interactions within the colchicine binding pocket. These compounds were examined in a tubulin polymerisation assay alongside the known tubulin polymerisation promotor, paclitaxel (69), and tubulin inhibitor, nocodazole (68). Of the analogues examined, indoles 43 and 47 were modest promotors of tubulin polymerisation, but less effective than paclitaxel. Analogues 39, 64, and 65 showed reduced microtubule formation consistent with tubulin inhibition. The variation in ring methoxy substituent with 50, 64 and 65, from o- to m- to p-, results in a concomitant reduction in cytotoxicity and a reduction in tubulin polymerisation, with p-OCH3 50 being the least active in this series of analogues. This presents 64 as a tubulin polymerisation inhibitor possessing novel chemotype and sub micromolar cytotoxicity. Naphthyl 39, with complete inhibition of tubulin polymerisation, gave rise to a sub 0.2 μM cell line cytotoxicity. Compounds 39 and 64 induced G2 + M cell cycle arrest indicative of inhibition of tubulin polymerisation, with 39 inducing an equivalent effect on cell cycle arrest as nocodazole (68).
中文翻译:

2,3-二氢喹唑啉-4(1H)-酮和喹唑啉-4(3H)-酮作为广谱细胞毒剂及其对微管蛋白聚合的影响
微管蛋白在有丝分裂中发挥核心作用,并且是包括紫杉醇在内的多种抗癌药物的靶标。本文合成了两个独立家族的2,3-二氢喹唑啉-4(1 H )-酮和喹唑啉-4(3 H )-酮,总共包含57种化合物。针对多种人类癌细胞系(HT29 结肠癌、U87 和 SJ-G2 胶质母细胞瘤、MCF-7 乳腺癌、A2780 卵巢细胞、H460 肺癌、A431 皮肤细胞、Du145 前列腺细胞、BE2-C 神经母细胞瘤和 MIA 胰腺细胞)的筛选揭示了这些类似物是广谱细胞毒性化合物。特别值得注意的是,2-苯乙烯基喹唑啉-4(3 H )-一51、2- (4-羟基苯乙烯基)喹唑啉-4(3 H )-一63、2- (2-甲氧基苯乙烯基)喹唑啉-4(3 H )-一种64和 2-(3-甲氧基苯乙烯基)喹唑啉-4(3 H )-一种65和 2-(萘-1-基)-2,3-二氢喹唑啉-4(1 H )-一种39表现出亚μM效力生长抑制值。其中 1-萘基39对 HT29、U87、A2780、H460 和 BE2-C 细胞系的活性 <50 nM。这些化合物的分子模型,例如2-(萘-1-基)-2,3-二氢喹唑啉-4(1 H )-一39、2-(2-甲氧基苯乙烯基)喹唑啉-4(3 H )-一64、 2-(3-甲氧基苯乙烯基)喹唑啉-4(3 H )-一65和 2-(4-甲氧基苯乙烯基)喹唑啉-4(3 H )-一50对接至已知的微管蛋白聚合抑制剂位点,突出显示了在秋水仙碱结合袋。这些化合物与已知的微管蛋白聚合促进剂紫杉醇 ( 69 ) 和微管蛋白抑制剂诺考达唑 ( 68 )一起在微管蛋白聚合测定中进行了检查。在所检查的类似物中,吲哚43和47是微管蛋白聚合的适度促进剂,但不如紫杉醇有效。类似物39、64和65显示微管形成减少,这与微管蛋白抑制一致。环甲氧基取代基50、64和65的变化,从o - 到m - 到p - ,导致细胞毒性同时降低,微管蛋白聚合减少,其中p -OCH 3 50是该系列中活性最低的的类似物。这呈现64 作为一种微管蛋白聚合抑制剂,具有新颖的化学型和亚微摩尔细胞毒性。 Naphthyl 39完全抑制微管蛋白聚合,产生低于 0.2 μM 的细胞系细胞毒性。化合物39和64诱导 G 2 + M 细胞周期停滞,表明抑制微管蛋白聚合,其中39诱导细胞周期停滞的效果与诺考达唑 ( 68 ) 相当。
更新日期:2024-03-06
中文翻译:

2,3-二氢喹唑啉-4(1H)-酮和喹唑啉-4(3H)-酮作为广谱细胞毒剂及其对微管蛋白聚合的影响
微管蛋白在有丝分裂中发挥核心作用,并且是包括紫杉醇在内的多种抗癌药物的靶标。本文合成了两个独立家族的2,3-二氢喹唑啉-4(1 H )-酮和喹唑啉-4(3 H )-酮,总共包含57种化合物。针对多种人类癌细胞系(HT29 结肠癌、U87 和 SJ-G2 胶质母细胞瘤、MCF-7 乳腺癌、A2780 卵巢细胞、H460 肺癌、A431 皮肤细胞、Du145 前列腺细胞、BE2-C 神经母细胞瘤和 MIA 胰腺细胞)的筛选揭示了这些类似物是广谱细胞毒性化合物。特别值得注意的是,2-苯乙烯基喹唑啉-4(3 H )-一51、2- (4-羟基苯乙烯基)喹唑啉-4(3 H )-一63、2- (2-甲氧基苯乙烯基)喹唑啉-4(3 H )-一种64和 2-(3-甲氧基苯乙烯基)喹唑啉-4(3 H )-一种65和 2-(萘-1-基)-2,3-二氢喹唑啉-4(1 H )-一种39表现出亚μM效力生长抑制值。其中 1-萘基39对 HT29、U87、A2780、H460 和 BE2-C 细胞系的活性 <50 nM。这些化合物的分子模型,例如2-(萘-1-基)-2,3-二氢喹唑啉-4(1 H )-一39、2-(2-甲氧基苯乙烯基)喹唑啉-4(3 H )-一64、 2-(3-甲氧基苯乙烯基)喹唑啉-4(3 H )-一65和 2-(4-甲氧基苯乙烯基)喹唑啉-4(3 H )-一50对接至已知的微管蛋白聚合抑制剂位点,突出显示了在秋水仙碱结合袋。这些化合物与已知的微管蛋白聚合促进剂紫杉醇 ( 69 ) 和微管蛋白抑制剂诺考达唑 ( 68 )一起在微管蛋白聚合测定中进行了检查。在所检查的类似物中,吲哚43和47是微管蛋白聚合的适度促进剂,但不如紫杉醇有效。类似物39、64和65显示微管形成减少,这与微管蛋白抑制一致。环甲氧基取代基50、64和65的变化,从o - 到m - 到p - ,导致细胞毒性同时降低,微管蛋白聚合减少,其中p -OCH 3 50是该系列中活性最低的的类似物。这呈现64 作为一种微管蛋白聚合抑制剂,具有新颖的化学型和亚微摩尔细胞毒性。 Naphthyl 39完全抑制微管蛋白聚合,产生低于 0.2 μM 的细胞系细胞毒性。化合物39和64诱导 G 2 + M 细胞周期停滞,表明抑制微管蛋白聚合,其中39诱导细胞周期停滞的效果与诺考达唑 ( 68 ) 相当。






































 京公网安备 11010802027423号
京公网安备 11010802027423号