当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Structural Characterization, and Biological Activities of 1,3,4- Thiadiazole Derivatives Containing Sulfonylpiperazine Structures
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2024-03-05 , DOI: 10.1002/cbdv.202400408 You-Hua Liu 1 , Fa-Li Wang 1 , Xiao-Li Ren 1 , Chang-Kun Li 1 , Lin-Hong Jin 1 , Xia Zhou 1
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2024-03-05 , DOI: 10.1002/cbdv.202400408 You-Hua Liu 1 , Fa-Li Wang 1 , Xiao-Li Ren 1 , Chang-Kun Li 1 , Lin-Hong Jin 1 , Xia Zhou 1
Affiliation
|
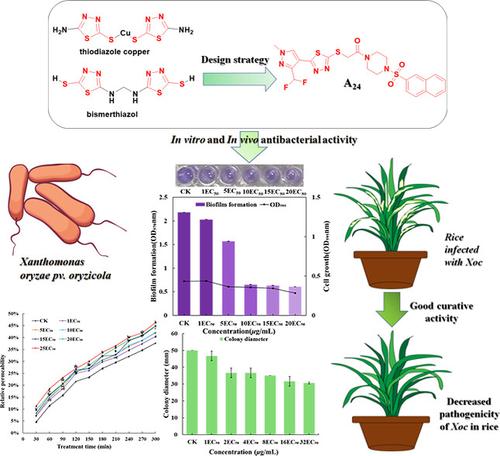
To develop novel bacterial biofilm inhibiting agents, a series of 1,3,4-thiadiazole derivatives containing sulfonylpiperazine structures were designed, synthesized, and characterized using 1H nuclear magnetic resonance (1H NMR), 13C nuclear magnetic resonance (13C NMR), and high-resolution mass spectrometry. Meanwhile, their biological activities were evaluated, and the ensuing structure–activity relationships were discussed. The bioassay results showed the substantial antimicrobial efficacy exhibited by most of the compounds. Among them, compound A24 demonstrated a strong efficacy with an EC50 value of 7.8 μg/mL in vitro against the Xanthomonas oryzae pv. oryzicola (Xoc) pathogen, surpassing commercial agents thiodiazole copper (31.8 μg/mL) and bismerthiazol (43.3 μg/mL). Mechanistic investigations into its anti-Xoc properties revealed that compound A24 operates by increasing the permeability of bacterial cell membranes, inhibiting biofilm formation and cell motility, and inducing morphological changes in bacterial cells. Importantly, in vivo tests showed its excellent protective and curative effects on rice bacterial leaf streak. Besides, molecular docking showed that the hydrophobic effect and hydrogen-bond interactions are key factors between the binding of A24 and AvrRxo1-ORF1. Therefore, these results suggest the utilization of 1,3,4-thiadiazole derivatives containing sulfonylpiperazine structures as a bacterial biofilm inhibiting agent, warranting further exploration in the realm of agrochemical development.
中文翻译:

含磺酰哌嗪结构的1,3,4-噻二唑衍生物的合成、结构表征及生物活性
为了开发新型细菌生物膜抑制剂,设计、合成了一系列含有磺酰哌嗪结构的1,3,4-噻二唑衍生物,并利用1 H 核磁共振( 1 H NMR)、 13 C 核磁共振( 13 C NMR)表征)和高分辨率质谱分析。同时,评估了它们的生物活性,并讨论了随之而来的构效关系。生物测定结果显示大多数化合物都表现出显着的抗菌功效。其中,化合物A 24在体外对米黄单胞菌表现出很强的功效,EC 50值为7.8 μg/mL。 oryzicola ( Xoc ) 病原体,超过了商业试剂噻二唑铜 (31.8 μg/mL) 和双甲噻唑 (43.3 μg/mL)。对其抗 Xoc 特性的机制研究表明,化合物 A 24通过增加细菌细胞膜的渗透性、抑制生物膜形成和细胞运动以及诱导细菌细胞形态变化来发挥作用。重要的是,体内试验表明其对水稻细菌性叶斑病具有优异的保护和治疗作用。此外,分子对接表明疏水效应和氢键相互作用是A 24与AvrRxo1-ORF1 结合的关键因素。因此,这些结果表明含有磺酰哌嗪结构的1,3,4-噻二唑衍生物可作为细菌生物膜抑制剂,值得在农用化学品开发领域进一步探索。
更新日期:2024-03-05
中文翻译:

含磺酰哌嗪结构的1,3,4-噻二唑衍生物的合成、结构表征及生物活性
为了开发新型细菌生物膜抑制剂,设计、合成了一系列含有磺酰哌嗪结构的1,3,4-噻二唑衍生物,并利用1 H 核磁共振( 1 H NMR)、 13 C 核磁共振( 13 C NMR)表征)和高分辨率质谱分析。同时,评估了它们的生物活性,并讨论了随之而来的构效关系。生物测定结果显示大多数化合物都表现出显着的抗菌功效。其中,化合物A 24在体外对米黄单胞菌表现出很强的功效,EC 50值为7.8 μg/mL。 oryzicola ( Xoc ) 病原体,超过了商业试剂噻二唑铜 (31.8 μg/mL) 和双甲噻唑 (43.3 μg/mL)。对其抗 Xoc 特性的机制研究表明,化合物 A 24通过增加细菌细胞膜的渗透性、抑制生物膜形成和细胞运动以及诱导细菌细胞形态变化来发挥作用。重要的是,体内试验表明其对水稻细菌性叶斑病具有优异的保护和治疗作用。此外,分子对接表明疏水效应和氢键相互作用是A 24与AvrRxo1-ORF1 结合的关键因素。因此,这些结果表明含有磺酰哌嗪结构的1,3,4-噻二唑衍生物可作为细菌生物膜抑制剂,值得在农用化学品开发领域进一步探索。






























 京公网安备 11010802027423号
京公网安备 11010802027423号