当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of α-Chloroamides via Photoenzymatic Hydroalkylation of Olefins
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-05 , DOI: 10.1021/jacs.4c00927
Yi Liu 1, 2 , Sophie G Bender 1, 2 , Damien Sorigue 1, 2, 3 , Daniel J Diaz 4, 5 , Andrew D Ellington 6 , Greg Mann 7 , Simon Allmendinger 7 , Todd K Hyster 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-05 , DOI: 10.1021/jacs.4c00927
Yi Liu 1, 2 , Sophie G Bender 1, 2 , Damien Sorigue 1, 2, 3 , Daniel J Diaz 4, 5 , Andrew D Ellington 6 , Greg Mann 7 , Simon Allmendinger 7 , Todd K Hyster 1, 2
Affiliation
|
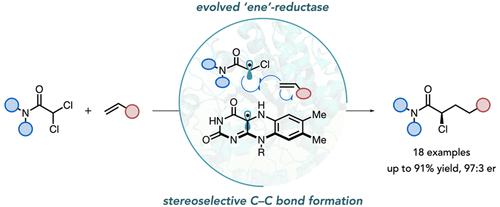
Photoenzymatic intermolecular hydroalkylations of olefins are highly enantioselective for chiral centers formed during radical termination but poorly selective for centers set in the C–C bond-forming event. Here, we report the evolution of a flavin-dependent “ene”-reductase to catalyze the coupling of α,α-dichloroamides with alkenes to afford α-chloroamides in good yield with excellent chemo- and stereoselectivity. These products can serve as linchpins in the synthesis of pharmaceutically valuable motifs. Mechanistic studies indicate that radical formation occurs by exciting a charge-transfer complex templated by the protein. Precise control over the orientation of molecules within the charge-transfer complex potentially accounts for the observed stereoselectivity. The work expands the types of motifs that can be prepared using photoenzymatic catalysis.
中文翻译:

通过烯烃的光酶加氢烷基化反应不对称合成 α-氯酰胺
烯烃的光酶分子间氢烷基化对自由基终止过程中形成的手性中心具有高度对映选择性,但对 C-C 键形成事件中设置的中心选择性较差。在这里,我们报道了一种黄素依赖性“烯”-还原酶的进化,以催化 α,α-二氯酰胺与烯烃的偶联,从而得到α-氯酰胺,产率高,具有优异的化学选择性和立体选择性。这些产品可以作为合成具有药学价值的基序的关键。机制研究表明,自由基的形成是通过激发蛋白质模板化的电荷转移复合物发生的。对电荷转移复合物内分子方向的精确控制可能是观察到的立体选择性的原因。这项工作扩展了可以使用光酶催化制备的基序类型。
更新日期:2024-03-05
中文翻译:

通过烯烃的光酶加氢烷基化反应不对称合成 α-氯酰胺
烯烃的光酶分子间氢烷基化对自由基终止过程中形成的手性中心具有高度对映选择性,但对 C-C 键形成事件中设置的中心选择性较差。在这里,我们报道了一种黄素依赖性“烯”-还原酶的进化,以催化 α,α-二氯酰胺与烯烃的偶联,从而得到α-氯酰胺,产率高,具有优异的化学选择性和立体选择性。这些产品可以作为合成具有药学价值的基序的关键。机制研究表明,自由基的形成是通过激发蛋白质模板化的电荷转移复合物发生的。对电荷转移复合物内分子方向的精确控制可能是观察到的立体选择性的原因。这项工作扩展了可以使用光酶催化制备的基序类型。


































 京公网安备 11010802027423号
京公网安备 11010802027423号