Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, biological activities, and evaluation molecular docking-dynamics studies of new phenylisoxazole quinoxalin-2-amine hybrids as potential α-amylase and α-glucosidase inhibitors
RSC Advances ( IF 3.9 ) Pub Date : 2024-03-05 , DOI: 10.1039/d3ra08642a Siti Nurshahira Mohd Radzuan 1 , Lacksany Phongphane 1 , Mohamad Hafizi Abu Bakar 2 , Mohammad Tasyriq Che Omar 3 , Nor Shafiqah Nor Shahril 2 , Unang Supratman 4 , Desi Harneti 4 , Habibah A. Wahab 5 , Mohamad Nurul Azmi 1
RSC Advances ( IF 3.9 ) Pub Date : 2024-03-05 , DOI: 10.1039/d3ra08642a Siti Nurshahira Mohd Radzuan 1 , Lacksany Phongphane 1 , Mohamad Hafizi Abu Bakar 2 , Mohammad Tasyriq Che Omar 3 , Nor Shafiqah Nor Shahril 2 , Unang Supratman 4 , Desi Harneti 4 , Habibah A. Wahab 5 , Mohamad Nurul Azmi 1
Affiliation
|
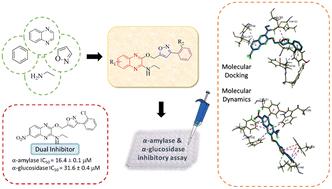
New phenylisoxazole quinoxalin-2-amine hybrids 5a–i were successfully synthesised with yields of 53–85% and characterised with various spectroscopy methods. The synthesised hybrids underwent in vitro α-amylase and α-glucosidase inhibitory assays, with acarbose as the positive control. Through the biological study, compound 5h exhibits the highest α-amylase inhibitory activity with IC50 = 16.4 ± 0.1 μM while compounds 5a–c, 5e and 5h exhibit great potential as α-glucosidase inhibitors, with 5c being the most potent (IC50 = 15.2 ± 0.3 μM). Among the compounds, 5h exhibits potential as a dual inhibitor for both α-amylase (IC50 = 16.4 ± 0.1 μM) and α-glucosidase (IC50 = 31.6 ± 0.4 μM) enzymes. Through the molecular docking studies, the inhibition potential of the selected compounds is supported. Compound 5h showed important interactions with α-amylase enzyme active sites and exhibited the highest binding energy of −8.9 ± 0.10 kcal mol−1, while compound 5c exhibited the highest binding energy of −9.0 ± 0.20 kcal mol−1 by forming important interactions with the α-glucosidase enzyme active sites. The molecular dynamics study showed that the selected compounds exhibited relative stability when binding with α-amylase and α-glucosidase enzymes. Additionally, compound 5h demonstrated a similar pattern of motion and mechanism of action as the commercially available miglitol.
中文翻译:

作为潜在α-淀粉酶和α-葡萄糖苷酶抑制剂的新型苯基异恶唑喹喔啉-2-胺杂化物的合成、生物活性和评估分子对接动力学研究
成功合成了新的苯基异恶唑喹喔啉-2-胺杂化物5a-i,产率为 53-85%,并用各种光谱方法进行了表征。合成的杂交体进行了体外α-淀粉酶和α-葡萄糖苷酶抑制测定,以阿卡波糖作为阳性对照。通过生物学研究,化合物5h显示出最高的 α-淀粉酶抑制活性,IC 50 = 16.4 ± 0.1 μM,而化合物5a–c、5e和5h显示出作为 α-葡萄糖苷酶抑制剂的巨大潜力,其中5c最为有效(IC 50 = 15.2 ± 0.3 μM)。在这些化合物中,5h显示出作为 α-淀粉酶 (IC 50 = 16.4 ± 0.1 μM) 和 α-葡萄糖苷酶 (IC 50 = 31.6 ± 0.4 μM) 双重抑制剂的潜力。通过分子对接研究,支持了所选化合物的抑制潜力。化合物5h表现出与α-淀粉酶活性位点的重要相互作用,并表现出最高结合能-8.9±0.10 kcal mol -1,而化合物5c通过与α-淀粉酶活性位点形成重要相互作用表现出最高结合能-9.0 ± 0.20 kcal mol -1 。 α-葡萄糖苷酶活性位点。分子动力学研究表明,所选化合物在与α-淀粉酶和α-葡萄糖苷酶结合时表现出相对稳定性。此外,化合物5h表现出与市售米格列醇相似的运动模式和作用机制。
更新日期:2024-03-05
中文翻译:

作为潜在α-淀粉酶和α-葡萄糖苷酶抑制剂的新型苯基异恶唑喹喔啉-2-胺杂化物的合成、生物活性和评估分子对接动力学研究
成功合成了新的苯基异恶唑喹喔啉-2-胺杂化物5a-i,产率为 53-85%,并用各种光谱方法进行了表征。合成的杂交体进行了体外α-淀粉酶和α-葡萄糖苷酶抑制测定,以阿卡波糖作为阳性对照。通过生物学研究,化合物5h显示出最高的 α-淀粉酶抑制活性,IC 50 = 16.4 ± 0.1 μM,而化合物5a–c、5e和5h显示出作为 α-葡萄糖苷酶抑制剂的巨大潜力,其中5c最为有效(IC 50 = 15.2 ± 0.3 μM)。在这些化合物中,5h显示出作为 α-淀粉酶 (IC 50 = 16.4 ± 0.1 μM) 和 α-葡萄糖苷酶 (IC 50 = 31.6 ± 0.4 μM) 双重抑制剂的潜力。通过分子对接研究,支持了所选化合物的抑制潜力。化合物5h表现出与α-淀粉酶活性位点的重要相互作用,并表现出最高结合能-8.9±0.10 kcal mol -1,而化合物5c通过与α-淀粉酶活性位点形成重要相互作用表现出最高结合能-9.0 ± 0.20 kcal mol -1 。 α-葡萄糖苷酶活性位点。分子动力学研究表明,所选化合物在与α-淀粉酶和α-葡萄糖苷酶结合时表现出相对稳定性。此外,化合物5h表现出与市售米格列醇相似的运动模式和作用机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号