当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Cascade of Strain-Driven Events Converting Benzynes to Alkynylbenzocyclobutenes to 1,3-Dien-5-ynes to Cyclic Allenes to Benzocyclohexadienones
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-04 , DOI: 10.1021/jacs.3c10225
Qian Xu 1 , Thomas R Hoye 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-03-04 , DOI: 10.1021/jacs.3c10225
Qian Xu 1 , Thomas R Hoye 1
Affiliation
|
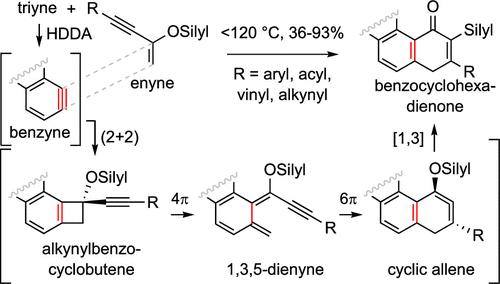
Here, we report a strain-promoted cascade reaction that proceeds via multiple strained intermediates, ultimately driven by the high potential energy inherent in alkyne triple bonds (C≡C). More specifically, four alkynes (three from an HDDA benzyne precursor and the fourth from a conjugated enyne reaction partner) are transformed into eight of the skeletal carbons in the benzocyclohexadienone products. The reaction pathway proceeds sequentially via strained benzyne, benzocyclobutene, and cyclic allene intermediates. DFT computations suggest that the slowest step following benzyne generation is the 4π-electrocyclic ring-opening of the alkynylbenzocyclobutene to a 1,3-dien-5-yne (an alkynylxylylene) intermediate. The activation energy for the subsequent 6π-electrocyclic ring-closure is lower than that for related acyclic dienynes because of the aromaticity that is being regained in the transition structure. Finally, the isolation of the benzocyclohexadienone products rather than their phenolic tautomers is notable.
中文翻译:

一系列应变驱动事件将苯炔转化为炔基苯并环丁烯、1,3-二烯-5-炔、环丙烯、苯并环己二酮
在这里,我们报告了一种应变促进的级联反应,该反应通过多个应变中间体进行,最终由炔三键(C=C)固有的高势能驱动。更具体地说,四种炔烃(三种来自 HDDA 苯炔前体,第四种来自共轭烯炔反应伙伴)被转化为苯并环己二烯酮产品中的八个骨架碳。该反应途径依次通过应变苯、苯并环丁烯和环状丙二烯中间体进行。 DFT 计算表明,生成苯炔后最慢的步骤是炔基苯并环丁烯向 1,3-二烯-5-炔(一种炔基二甲苯)中间体进行 4π 电环开环。由于过渡结构中芳香性的恢复,后续 6π-电环闭环的活化能低于相关无环二烯的活化能。最后,值得注意的是苯并环己二烯酮产物而不是它们的酚类互变异构体的分离。
更新日期:2024-03-04
中文翻译:

一系列应变驱动事件将苯炔转化为炔基苯并环丁烯、1,3-二烯-5-炔、环丙烯、苯并环己二酮
在这里,我们报告了一种应变促进的级联反应,该反应通过多个应变中间体进行,最终由炔三键(C=C)固有的高势能驱动。更具体地说,四种炔烃(三种来自 HDDA 苯炔前体,第四种来自共轭烯炔反应伙伴)被转化为苯并环己二烯酮产品中的八个骨架碳。该反应途径依次通过应变苯、苯并环丁烯和环状丙二烯中间体进行。 DFT 计算表明,生成苯炔后最慢的步骤是炔基苯并环丁烯向 1,3-二烯-5-炔(一种炔基二甲苯)中间体进行 4π 电环开环。由于过渡结构中芳香性的恢复,后续 6π-电环闭环的活化能低于相关无环二烯的活化能。最后,值得注意的是苯并环己二烯酮产物而不是它们的酚类互变异构体的分离。

































 京公网安备 11010802027423号
京公网安备 11010802027423号