当前位置:
X-MOL 学术
›
Nano Today
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of tumor-cell nucleus targeting lanthanide nano-prodrugs with lutetium-177 labelling for high-efficient tumor fluorescence-localization and radionuclide therapy
Nano Today ( IF 13.2 ) Pub Date : 2024-02-28 , DOI: 10.1016/j.nantod.2024.102214
Zhifen Wu , Hao Chen , Hongyun Zhang , Lixiang Ye , Jianxi Ke , Yongsheng Liu , Pengming Sun , Maochun Hong
Nano Today ( IF 13.2 ) Pub Date : 2024-02-28 , DOI: 10.1016/j.nantod.2024.102214
Zhifen Wu , Hao Chen , Hongyun Zhang , Lixiang Ye , Jianxi Ke , Yongsheng Liu , Pengming Sun , Maochun Hong
|
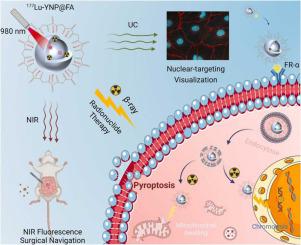
Tumor-cell nucleus targeting is highly desired for theragnostic nano-prodrugs (NPDs) to enhance cancer diagnostic and therapeutic efficacy as compared to those targeting the cytoplasm or other intracellular organelles. This study presents a well-designed tumor-cell nucleus targeting theragnostic NPD called Lu-YNP@FA that can not only deliver bright upconversion/NIR-II fluorescence but also emit radioactive β-ray radiation for efficient tumor fluorescence localization and radionuclide therapy, based on PEGylated folic acid (FA) decorated NaYF:Yb/Er@NaYF core-shell nanocrystals with radionuclide lutetium-177 labeling. Owing to their unique tumor-cell nucleus targeting capacity, the well-designed Lu-YNP@FA NPDs can rapidly target the nuclei of Hela cells within eight hours, thereby allowing for the precise localization of two-hundred-micron-sized metastatic tumors of cervical carcinoma, even in the abdominal cavity of a living mouse model, through NIR-II fluorescence imaging. Importantly, these Lu-YNP@FA NPDs exhibit superior tumor accumulation (∼24.6%) and retention (∼7.1 days) compared to the NPDs without tumor-cell nucleus targeting ability. This results in highly efficient anticancer outcomes, both and , through a pyroptosis-mediated cell death associated with intracellular β-ray radiation of Lu radionuclide. These findings have significant implications for the intelligent design of organelle-specific targeting theranostic NPDs, offering new options for diagnosis and treatment in radiopharmaceutical therapy of cancer.
中文翻译:

构建镥177标记的靶向镧系纳米前药的肿瘤细胞核,用于高效肿瘤荧光定位和放射性核素治疗
与靶向细胞质或其他细胞内细胞器的纳米前药(NPD)相比,肿瘤细胞核靶向是非常需要的,以增强癌症诊断和治疗功效。这项研究提出了一种精心设计的靶向治疗诊断 NPD 的肿瘤细胞核,称为 Lu-YNP@FA,它不仅可以提供明亮的上转换/NIR-II 荧光,还可以发射放射性 β 射线辐射,用于有效的肿瘤荧光定位和放射性核素治疗。聚乙二醇化叶酸(FA)修饰的带有放射性核素镥177标记的NaYF:Yb/Er@NaYF核壳纳米晶体。由于其独特的肿瘤细胞核靶向能力,精心设计的Lu-YNP@FA NPDs可以在8小时内快速靶向Hela细胞的细胞核,从而实现对200微米大小的转移性肿瘤的精确定位。通过 NIR-II 荧光成像,即使在活体小鼠模型的腹腔中,也能发现宫颈癌。重要的是,与没有肿瘤细胞核靶向能力的 NPD 相比,这些 Lu-YNP@FA NPD 表现出优异的肿瘤积累(~24.6%)和保留(~7.1 天)。这通过与细胞内 Lu 放射性核素的 β 射线辐射相关的细胞焦亡介导的细胞死亡,产生高效的抗癌结果。这些发现对于细胞器特异性靶向治疗诊断 NPD 的智能设计具有重要意义,为癌症放射性药物治疗的诊断和治疗提供了新的选择。
更新日期:2024-02-28
中文翻译:

构建镥177标记的靶向镧系纳米前药的肿瘤细胞核,用于高效肿瘤荧光定位和放射性核素治疗
与靶向细胞质或其他细胞内细胞器的纳米前药(NPD)相比,肿瘤细胞核靶向是非常需要的,以增强癌症诊断和治疗功效。这项研究提出了一种精心设计的靶向治疗诊断 NPD 的肿瘤细胞核,称为 Lu-YNP@FA,它不仅可以提供明亮的上转换/NIR-II 荧光,还可以发射放射性 β 射线辐射,用于有效的肿瘤荧光定位和放射性核素治疗。聚乙二醇化叶酸(FA)修饰的带有放射性核素镥177标记的NaYF:Yb/Er@NaYF核壳纳米晶体。由于其独特的肿瘤细胞核靶向能力,精心设计的Lu-YNP@FA NPDs可以在8小时内快速靶向Hela细胞的细胞核,从而实现对200微米大小的转移性肿瘤的精确定位。通过 NIR-II 荧光成像,即使在活体小鼠模型的腹腔中,也能发现宫颈癌。重要的是,与没有肿瘤细胞核靶向能力的 NPD 相比,这些 Lu-YNP@FA NPD 表现出优异的肿瘤积累(~24.6%)和保留(~7.1 天)。这通过与细胞内 Lu 放射性核素的 β 射线辐射相关的细胞焦亡介导的细胞死亡,产生高效的抗癌结果。这些发现对于细胞器特异性靶向治疗诊断 NPD 的智能设计具有重要意义,为癌症放射性药物治疗的诊断和治疗提供了新的选择。































 京公网安备 11010802027423号
京公网安备 11010802027423号