当前位置:
X-MOL 学术
›
Mater. Horiz.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly coordinative molecular cobalt–phthalocyanine electrocatalyst on an oxidized single-walled carbon nanotube for efficient hydrogen peroxide production
Materials Horizons ( IF 12.2 ) Pub Date : 2024-03-05 , DOI: 10.1039/d3mh02142d Yaoxin Li 1, 2 , Haoying Cheng 2, 3 , Meilin Wang 2, 3 , Jiaoxing Xu 1, 2 , Lunhui Guan 1, 2
Materials Horizons ( IF 12.2 ) Pub Date : 2024-03-05 , DOI: 10.1039/d3mh02142d Yaoxin Li 1, 2 , Haoying Cheng 2, 3 , Meilin Wang 2, 3 , Jiaoxing Xu 1, 2 , Lunhui Guan 1, 2
Affiliation
|
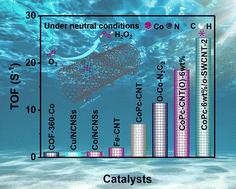
H2O2 production via the two-electron oxygen reduction reaction (2e− ORR) offers a potential alternative to the current anthraquinone method owing to its efficiency and environmental friendliness. However, it is necessary to determine the structures of electrocatalysts with cost-effectiveness and high efficiency for future industrialization demand. Herein, a supramolecular catalyst composed of cobalt–phthalocyanine on a near-monodispersed and oxidized single-walled carbon nanotube (CoPc/o-SWCNT) was synthesized via a solution self-assembly method for catalyzing the 2e− ORR for H2O2 electrosynthesis. Benefiting from the enhanced intermolecular interaction by introducing oxygen functional groups on o-SWCNTs, the oxidation states of single-atom Co sites were tuned via the formation of two extra Co–O bonds. Coupled with structural characterizations, density-functional theory (DFT) calculations reveal that the depressed d-band center of the Co site regulated by two axially-bridged O atoms gives rise to a suitable binding strength of oxygen intermediates (*OOH) to favor the 2e− ORR. Thus, the CoPc-6wt%/o-SWCNT-2 catalyst with optimized synthetic parameters delivers competitive 2e− ORR performance for H2O2 electrosynthesis in a neutral electrolyte (pH = 7), including enhanced H2O2 generation, satisfactory molar selectivity of ∼83–95% and long-period stability (75 h) in H-cell measurement. Moreover, it could also be boosted to show a high current of 45 mA cm−2, recorded turnover frequency of 25.3 ± 0.5 s−1 and maximum H2O2 production rate of 5.85 mol g−1 h−1 with a continuous H2O2 accumulation of 1.2 wt% in a flow-cell device, which outperformed most of the reported neutral-selective nonprecious metal single-atom catalysts.
中文翻译:

氧化单壁碳纳米管上的高度配位分子钴酞菁电催化剂用于高效生产过氧化氢
通过双电子氧还原反应 (2e – ORR) 生产 H 2 O 2因其高效且环境友好而成为当前蒽醌方法的潜在替代方案。然而,有必要确定具有成本效益和高效率的电催化剂结构以满足未来工业化的需求。在此,通过溶液自组装方法合成了由钴酞菁组成的近单分散氧化单壁碳纳米管(CoPc/o-SWCNT)超分子催化剂,用于催化2e - ORR电合成H 2 O 2 。通过在 o-SWCNT 上引入氧官能团来增强分子间相互作用,通过形成两个额外的 Co-O 键来调节单原子 Co 位点的氧化态。结合结构表征,密度泛函理论 (DFT) 计算表明,由两个轴向桥接的 O 原子调节的 Co 位点的凹陷 d 带中心产生了合适的氧中间体 (*OOH) 结合强度,有利于2e - ORR。 因此,具有优化合成参数的CoPc-6wt%/o-SWCNT-2催化剂为中性电解质(pH = 7)中的H 2 O 2电合成提供了具有竞争力的2e − ORR性能,包括增强的H 2 O 2生成、令人满意的摩尔比H 细胞测量中的选择性约为 83–95% 且具有长期稳定性(75 小时)。此外,它还可以增强以显示45 mA cm -2的高电流,记录的周转频率为25.3 ± 0.5 s -1 ,并且在连续H 的情况下最大H 2 O 2生产率为5.85 mol g -1 h -1 2 O 2在流通池装置中积累了1.2 wt%,其性能优于大多数已报道的中性选择性非贵金属单原子催化剂。
更新日期:2024-03-05
中文翻译:

氧化单壁碳纳米管上的高度配位分子钴酞菁电催化剂用于高效生产过氧化氢
通过双电子氧还原反应 (2e – ORR) 生产 H 2 O 2因其高效且环境友好而成为当前蒽醌方法的潜在替代方案。然而,有必要确定具有成本效益和高效率的电催化剂结构以满足未来工业化的需求。在此,通过溶液自组装方法合成了由钴酞菁组成的近单分散氧化单壁碳纳米管(CoPc/o-SWCNT)超分子催化剂,用于催化2e - ORR电合成H 2 O 2 。通过在 o-SWCNT 上引入氧官能团来增强分子间相互作用,通过形成两个额外的 Co-O 键来调节单原子 Co 位点的氧化态。结合结构表征,密度泛函理论 (DFT) 计算表明,由两个轴向桥接的 O 原子调节的 Co 位点的凹陷 d 带中心产生了合适的氧中间体 (*OOH) 结合强度,有利于2e - ORR。 因此,具有优化合成参数的CoPc-6wt%/o-SWCNT-2催化剂为中性电解质(pH = 7)中的H 2 O 2电合成提供了具有竞争力的2e − ORR性能,包括增强的H 2 O 2生成、令人满意的摩尔比H 细胞测量中的选择性约为 83–95% 且具有长期稳定性(75 小时)。此外,它还可以增强以显示45 mA cm -2的高电流,记录的周转频率为25.3 ± 0.5 s -1 ,并且在连续H 的情况下最大H 2 O 2生产率为5.85 mol g -1 h -1 2 O 2在流通池装置中积累了1.2 wt%,其性能优于大多数已报道的中性选择性非贵金属单原子催化剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号