当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
From Serendipity to Precision: Decoding the Enigma of Rearrangement in Scholl-Type Reactions for Programmable Cyclization
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-02-29 , DOI: 10.1021/acs.joc.3c02050 Nagaraju Ponugoti 1 , Sudhakar Maddala 1 , Parthasarathy Venkatakrishnan 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-02-29 , DOI: 10.1021/acs.joc.3c02050 Nagaraju Ponugoti 1 , Sudhakar Maddala 1 , Parthasarathy Venkatakrishnan 1
Affiliation
|
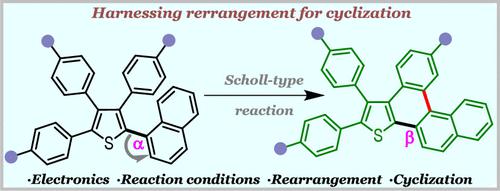
Rearrangements in the Scholl reaction have traditionally been serendipitous, lacking a systematic approach for synthesizing rearranged and cyclized products. This paper introduces a strategic pathway to achieve rearranged-cyclized thienotetrahelicene derivatives over direct-cyclized chrysenothiophene derivatives by finely modifying the reaction conditions and tuning the electronic properties in Scholl-type reaction precursors, tetraarylthiophenes. Through careful design principles, we demonstrate the programmable synthesis of these distinct products.
中文翻译:

从偶然性到精确性:破解 Scholl 型反应中可编程环化重排之谜
Scholl 反应中的重排传统上是偶然的,缺乏合成重排和环化产物的系统方法。本文介绍了一种通过精细修改反应条件和调整 Scholl 型反应前体四芳基噻吩的电子性质,在直接环化并噻吩衍生物上实现重排环化噻吩四螺旋衍生物的战略途径。通过仔细的设计原则,我们展示了这些不同产品的可编程综合。
更新日期:2024-02-29
中文翻译:

从偶然性到精确性:破解 Scholl 型反应中可编程环化重排之谜
Scholl 反应中的重排传统上是偶然的,缺乏合成重排和环化产物的系统方法。本文介绍了一种通过精细修改反应条件和调整 Scholl 型反应前体四芳基噻吩的电子性质,在直接环化并噻吩衍生物上实现重排环化噻吩四螺旋衍生物的战略途径。通过仔细的设计原则,我们展示了这些不同产品的可编程综合。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号