当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Access to Tetrahydrothiopyrano[2,3-b]Indole Derivatives via Zinc-Catalyzed Asymmetric [3+3] Annulation of Indoline-2-Thiones with Yne–Enones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-02-29 , DOI: 10.1002/adsc.202400072 Dan-Dan Cui 1 , Jian-Wen Shi 1 , Tong Wang 1 , Yuan-Zhao Hua 2 , mincan wang 1 , Guang-Jian Mei 2 , Jun-Long Niu 1 , Shikun Jia 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-02-29 , DOI: 10.1002/adsc.202400072 Dan-Dan Cui 1 , Jian-Wen Shi 1 , Tong Wang 1 , Yuan-Zhao Hua 2 , mincan wang 1 , Guang-Jian Mei 2 , Jun-Long Niu 1 , Shikun Jia 1
Affiliation
|
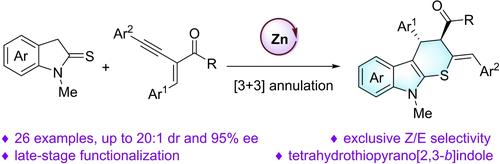
We report herein an enantioselective [3 + 3] annulation of indoline‐2‐thiones with yne−enones by chiral dinuclear zinc catalysts via a Brønsted base and Lewis acid cooperative activation. This transformation proceeded through sequential conjugate addition, allenyl ketone formation and intramolecular sulfa‐Michael 6‐endo‐trig cyclization. A range of enantioenriched tetrahydrothiopyrano[2,3‐b]indole derivatives bearing an exocyclic double bond were obtained in moderate yields with excellent stereoselectivities (up to 20 : 1 dr, 20:1 Z/E ratio and 95% ee). Late‐stage functionalization, large‐scale experiment and further derivatizations were also explored.
中文翻译:

通过锌催化吲哚啉-2-硫酮与炔烯酮的不对称[3+3]环化反应获得四氢吡喃并[2,3-b]吲哚衍生物
我们在此报告了通过手性双核锌催化剂通过布朗斯台德碱和路易斯酸协同活化,二氢吲哚-2-硫酮与炔烯酮的对映选择性[3 + 3]环化。这种转化通过顺序共轭加成、丙二烯基酮形成和分子内磺胺-Michael 6-endo-trig环化进行。以中等产率获得了一系列带有环外双键的对映体富集四氢吡喃并[2,3-b]吲哚衍生物,具有优异的立体选择性(高达 20:1 dr、20:1 Z/E 比和 95% ee)。还探索了后期功能化、大规模实验和进一步衍生化。
更新日期:2024-02-29
中文翻译:

通过锌催化吲哚啉-2-硫酮与炔烯酮的不对称[3+3]环化反应获得四氢吡喃并[2,3-b]吲哚衍生物
我们在此报告了通过手性双核锌催化剂通过布朗斯台德碱和路易斯酸协同活化,二氢吲哚-2-硫酮与炔烯酮的对映选择性[3 + 3]环化。这种转化通过顺序共轭加成、丙二烯基酮形成和分子内磺胺-Michael 6-endo-trig环化进行。以中等产率获得了一系列带有环外双键的对映体富集四氢吡喃并[2,3-b]吲哚衍生物,具有优异的立体选择性(高达 20:1 dr、20:1 Z/E 比和 95% ee)。还探索了后期功能化、大规模实验和进一步衍生化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号