当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Imaging of GGT and HOBr-Triggered Atherosclerotic Plaque Rupture via Activating the RunX2/Col IV Signaling Pathway
Analytical Chemistry ( IF 6.7 ) Pub Date : 2024-03-01 , DOI: 10.1021/acs.analchem.3c05073
Xiaoqing Huang 1 , Shengyue Zhang 1 , Wei Fu 2 , Liping Wang 1 , Zhenhua Liu 1 , Yue Tang 3 , Wen Gao 1 , Bo Tang 1, 4
Analytical Chemistry ( IF 6.7 ) Pub Date : 2024-03-01 , DOI: 10.1021/acs.analchem.3c05073
Xiaoqing Huang 1 , Shengyue Zhang 1 , Wei Fu 2 , Liping Wang 1 , Zhenhua Liu 1 , Yue Tang 3 , Wen Gao 1 , Bo Tang 1, 4
Affiliation
|
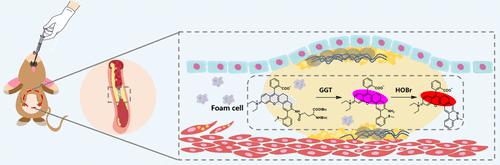
Calcification and abnormal collagen deposition within blood vessels constitute causative factors for atherosclerotic plaque rupture, and their occurrence is intimately linked with γ-glutamyltranspeptidase (GGT) and hypobromous acid (HOBr). However, the underlying regulatory mechanisms of GGT and HOBr in plaque rupture remain unclear. Hence, we developed a dual-responsive near-infrared (NIR) fluorescent probe (BOC-H) that effectively avoids spectral crosstalk for the in situ visualization of the fluctuations in GGT and HOBr levels during atherosclerotic plaque rupture. We found that both GGT and HOBr contents increase significantly in the calcification models of cells and animals. The overexpressed GGT participated in intracellular oxygen-promoting behavior, which obviously upregulated the expression of RunX2 and Col IV by facilitating H2O2 and HOBr secretion. This process triggered calcification and abnormal collagen deposition within the plaque, which raised the risk of plaque rupture. PM2.5-induced arteriosclerotic calcification models further verified the results that GGT and HOBr accelerate plaque rupture via activation of the RunX2/Col IV signaling pathway. Moreover, the assessment of GGT and HOBr in serum samples from patients with acute myocardial infarction further confirmed the co-regulation of GGT and HOBr in the plaque rupture. Together, our studies highlight the involvement of GGT and HOBr in driving plaque rupture and offer new targets for the prevention and treatment of acute cardiovascular disease.
中文翻译:

通过激活 RunX2/Col IV 信号通路对 GGT 和 HOBr 触发的动脉粥样硬化斑块破裂进行原位成像
血管内钙化和异常胶原沉积是动脉粥样硬化斑块破裂的致病因素,其发生与γ-谷氨酰转肽酶(GGT)和次溴酸(HOBr)密切相关。然而,GGT 和 HOBr 在斑块破裂中的潜在调节机制仍不清楚。因此,我们开发了一种双响应近红外 (NIR) 荧光探针 (BOC-H),可有效避免光谱串扰,用于动脉粥样硬化斑块破裂期间 GGT 和 HOBr 水平波动的原位可视化。我们发现细胞和动物钙化模型中 GGT 和 HOBr 含量均显着增加。过表达的GGT参与细胞内促氧行为,通过促进H 2 O 2和HOBr的分泌,明显上调RunX2和Col IV的表达。这一过程引发了斑块内的钙化和异常的胶原沉积,从而增加了斑块破裂的风险。 PM2.5诱导的动脉硬化钙化模型进一步验证了GGT和HOBr通过激活RunX2/Col IV信号通路加速斑块破裂的结果。此外,对急性心肌梗死患者血清样本中GGT和HOBr的评估进一步证实了GGT和HOBr在斑块破裂中的共同调节作用。总之,我们的研究强调了 GGT 和 HOBr 在驱动斑块破裂中的作用,并为预防和治疗急性心血管疾病提供了新的靶点。
更新日期:2024-03-01
中文翻译:

通过激活 RunX2/Col IV 信号通路对 GGT 和 HOBr 触发的动脉粥样硬化斑块破裂进行原位成像
血管内钙化和异常胶原沉积是动脉粥样硬化斑块破裂的致病因素,其发生与γ-谷氨酰转肽酶(GGT)和次溴酸(HOBr)密切相关。然而,GGT 和 HOBr 在斑块破裂中的潜在调节机制仍不清楚。因此,我们开发了一种双响应近红外 (NIR) 荧光探针 (BOC-H),可有效避免光谱串扰,用于动脉粥样硬化斑块破裂期间 GGT 和 HOBr 水平波动的原位可视化。我们发现细胞和动物钙化模型中 GGT 和 HOBr 含量均显着增加。过表达的GGT参与细胞内促氧行为,通过促进H 2 O 2和HOBr的分泌,明显上调RunX2和Col IV的表达。这一过程引发了斑块内的钙化和异常的胶原沉积,从而增加了斑块破裂的风险。 PM2.5诱导的动脉硬化钙化模型进一步验证了GGT和HOBr通过激活RunX2/Col IV信号通路加速斑块破裂的结果。此外,对急性心肌梗死患者血清样本中GGT和HOBr的评估进一步证实了GGT和HOBr在斑块破裂中的共同调节作用。总之,我们的研究强调了 GGT 和 HOBr 在驱动斑块破裂中的作用,并为预防和治疗急性心血管疾病提供了新的靶点。

































 京公网安备 11010802027423号
京公网安备 11010802027423号