当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
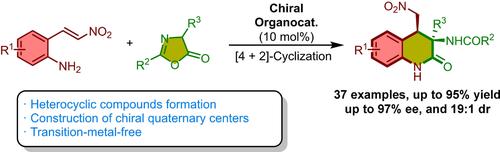
Asymmetric Synthesis of 3,4-Dihydroquinolin-2-ones via Organocatalytic [4+2]-Cyclization of 2-Amino-β-nitrostyrenes with Azlactones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-02-28 , DOI: 10.1002/adsc.202400061 Heebum Kim 1 , Yeongju Kim 1 , Sung-Gon Kim 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-02-28 , DOI: 10.1002/adsc.202400061 Heebum Kim 1 , Yeongju Kim 1 , Sung-Gon Kim 1
Affiliation
|
Dihydroquinolin‐2‐ones, recognized for their significant bioactive properties, feature a unique six‐membered structure with nitrogen‐containing heterocycles. A breakthrough method has been developed to synthesize enantioenriched 3,4‐dihydroquinoline‐2‐one derivatives. This innovative approach utilizes an asymmetric [4+2]‐cyclization process, combining 2‐amino‐β‐nitrostyrenes with azlactones, and is facilitated by a bifunctional squaramide‐based organocatalyst. This innovative approach has enabled the creation of novel chiral 3,4‐dihydroquinoline‐2‐ones with complex structures, including chiral quaternary centers. The process is remarkably efficient, delivering high yields (up to 91%), excellent enantiomeric excess (up to 97% ee), and superior diastereoselectivity (up to 19:1 dr).
中文翻译:

2-氨基-β-硝基苯乙烯与吖内酯有机催化[4+2]-环化不对称合成3,4-二氢喹啉-2-酮
二氢喹啉-2-酮因其显着的生物活性而被认可,具有独特的六元结构和含氮杂环。开发了一种突破性方法来合成对映体富集的 3,4-二氢喹啉-2-酮衍生物。这种创新方法利用不对称[4+2]环化过程,将2-氨基-β-硝基苯乙烯与吖内酯结合,并通过双功能方酰胺基有机催化剂促进。这种创新方法使得能够创建具有复杂结构(包括手性四级中心)的新型手性 3,4-二氢喹啉-2-酮。该工艺非常高效,可实现高产率(高达 91%)、出色的对映体过量(高达 97% ee)和卓越的非对映选择性(高达 19:1 dr)。
更新日期:2024-02-28
中文翻译:

2-氨基-β-硝基苯乙烯与吖内酯有机催化[4+2]-环化不对称合成3,4-二氢喹啉-2-酮
二氢喹啉-2-酮因其显着的生物活性而被认可,具有独特的六元结构和含氮杂环。开发了一种突破性方法来合成对映体富集的 3,4-二氢喹啉-2-酮衍生物。这种创新方法利用不对称[4+2]环化过程,将2-氨基-β-硝基苯乙烯与吖内酯结合,并通过双功能方酰胺基有机催化剂促进。这种创新方法使得能够创建具有复杂结构(包括手性四级中心)的新型手性 3,4-二氢喹啉-2-酮。该工艺非常高效,可实现高产率(高达 91%)、出色的对映体过量(高达 97% ee)和卓越的非对映选择性(高达 19:1 dr)。







































 京公网安备 11010802027423号
京公网安备 11010802027423号