Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced Retention of Cd(II) by Exfoliated Bentonite and Its Methoxy Form: Steric and Energetic Studies
ACS Omega ( IF 3.7 ) Pub Date : 2024-02-29 , DOI: 10.1021/acsomega.3c08592 Mostafa R Abukhadra 1, 2 , Nourhan Nasser 1, 2 , Ahmed M El-Sherbeeny 3 , Wail Al Zoubi 4
ACS Omega ( IF 3.7 ) Pub Date : 2024-02-29 , DOI: 10.1021/acsomega.3c08592 Mostafa R Abukhadra 1, 2 , Nourhan Nasser 1, 2 , Ahmed M El-Sherbeeny 3 , Wail Al Zoubi 4
Affiliation
|
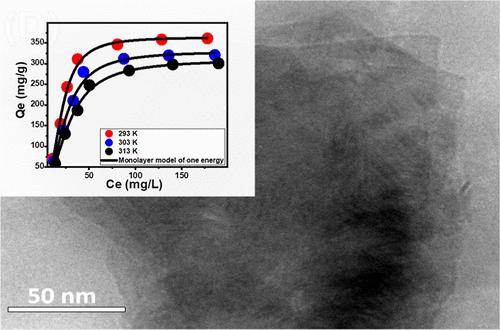
Synergistic studies were conducted to evaluate the retention potentiality of exfoliating bentonite (EXBEN) as well as its methanol hybridization derivative (Mth/EXBEN) toward Cd(II) ions to be able to verify the effects of the transformation processes. The adsorption characteristics were established by considering the steric and energetic aspects of the implemented advanced equilibrium simulation, specifically the monolayer model with a single energy level. Throughout the full saturation states, the adsorption characteristics of Cd(II) increased substantially to 363.7 mg/g following the methanol hybridized treatment in comparison to EXBEN (293.2 mg/g) as well as raw bentonite (BEN) (187.3 mg/g). The steric analysis indicated a significant rise in the levels of the active sites following the exfoliation procedure [retention site density (Nm) = 162.96 mg/g] and the chemical modification with methanol [retention site density (Nm) = 157.1 mg/g]. These findings clarify the improvement in the potential of Mth/EXBEN to eliminate Cd(II). Furthermore, each open site of Mth/EXBEN has the capacity to bind approximately three ions of Cd(II) in a vertically aligned manner. The energetic investigations, encompassing the Gaussian energy (less than 8 kJ/mol) plus the adsorption energy (less than 40 kJ/mol), provide evidence of the physical sequestration of Cd(II). This process may involve the collaborative impacts of dipole binding forces (ranging from 2 to 29 kJ/mol) and hydrogen binding (less than 30 kJ/mol). The measurable thermodynamic functions, particularly entropy, internal energy, and free enthalpy, corroborate the exothermic and spontaneous nature of Cd(II) retention by Mth/EXBEN, as opposed to those by EXBEN and BE.
中文翻译:

剥落膨润土及其甲氧基形式增强对 Cd(II) 的保留:空间和能量研究
进行了协同研究来评估剥离膨润土 (EXBEN) 及其甲醇杂化衍生物 (Mth/EXBEN) 对 Cd(II) 离子的保留潜力,以便能够验证转化过程的效果。通过考虑所实施的高级平衡模拟的空间和能量方面,特别是具有单一能级的单层模型,建立了吸附特性。在整个完全饱和状态下,与 EXBEN (293.2 mg/g) 以及原始膨润土 (BEN) (187.3 mg/g) 相比,甲醇杂化处理后 Cd(II) 的吸附特性大幅增加至 363.7 mg/g 。空间分析表明,在剥离过程[保留位点密度( N m )= 162.96 mg/g]和甲醇化学修饰[保留位点密度( N m )= 157.1 mg/g]之后,活性位点的水平显着升高。克]。这些发现阐明了 Mth/EXBEN 消除 Cd(II) 潜力的提高。此外,Mth/EXBEN 的每个开放位点能够以垂直排列的方式结合大约三个 Cd(II) 离子。能量研究,包括高斯能量(小于 8 kJ/mol)加上吸附能(小于 40 kJ/mol),提供了 Cd(II) 物理隔离的证据。该过程可能涉及偶极结合力(范围从 2 至 29 kJ/mol)和氢结合力(小于 30 kJ/mol)的协同影响。 可测量的热力学函数,特别是熵、内能和自由焓,证实了 Mth/EXBEN 保留 Cd(II) 的放热和自发性质,而不是 EXBEN 和 BE 的保留。
更新日期:2024-02-29
中文翻译:

剥落膨润土及其甲氧基形式增强对 Cd(II) 的保留:空间和能量研究
进行了协同研究来评估剥离膨润土 (EXBEN) 及其甲醇杂化衍生物 (Mth/EXBEN) 对 Cd(II) 离子的保留潜力,以便能够验证转化过程的效果。通过考虑所实施的高级平衡模拟的空间和能量方面,特别是具有单一能级的单层模型,建立了吸附特性。在整个完全饱和状态下,与 EXBEN (293.2 mg/g) 以及原始膨润土 (BEN) (187.3 mg/g) 相比,甲醇杂化处理后 Cd(II) 的吸附特性大幅增加至 363.7 mg/g 。空间分析表明,在剥离过程[保留位点密度( N m )= 162.96 mg/g]和甲醇化学修饰[保留位点密度( N m )= 157.1 mg/g]之后,活性位点的水平显着升高。克]。这些发现阐明了 Mth/EXBEN 消除 Cd(II) 潜力的提高。此外,Mth/EXBEN 的每个开放位点能够以垂直排列的方式结合大约三个 Cd(II) 离子。能量研究,包括高斯能量(小于 8 kJ/mol)加上吸附能(小于 40 kJ/mol),提供了 Cd(II) 物理隔离的证据。该过程可能涉及偶极结合力(范围从 2 至 29 kJ/mol)和氢结合力(小于 30 kJ/mol)的协同影响。 可测量的热力学函数,特别是熵、内能和自由焓,证实了 Mth/EXBEN 保留 Cd(II) 的放热和自发性质,而不是 EXBEN 和 BE 的保留。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号