当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of (−)-Enigmazole A by the Macrocyclization/Transannular Pyran Cyclization Strategy
Organic Letters ( IF 4.9 ) Pub Date : 2024-02-29 , DOI: 10.1021/acs.orglett.4c00290 Taisei Masuda 1 , Kyoya Ohyama 1 , Atsushi Yoshimura 1 , Haruhiko Fuwa 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-02-29 , DOI: 10.1021/acs.orglett.4c00290 Taisei Masuda 1 , Kyoya Ohyama 1 , Atsushi Yoshimura 1 , Haruhiko Fuwa 1
Affiliation
|
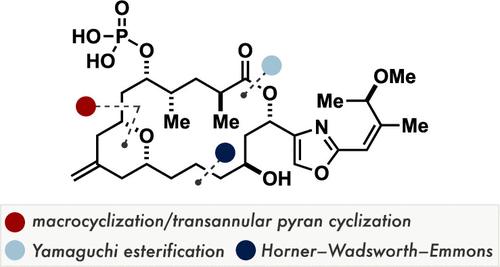
An 18-step synthesis of (−)-enigmazole A is herein disclosed. The present synthesis is based on a modular assembly of three building blocks of similar complexity, a macrocyclic ring-closing metathesis to forge the 18-membered macrocyclic skeleton, and a desilylative transannular oxa-Michael addition for stereoselective construction of the 2,6-cis-substituted tetrahydropyran ring.
中文翻译:

大环化/跨环吡喃环化策略全合成 (−)-Enigmodium A
本文公开了(-)-恩尼格唑A的18步合成。本合成基于三个具有相似复杂性的构件的模块化组装、用于形成18元大环骨架的大环闭环复分解、以及用于立体选择性构建2,6-顺式的脱甲硅烷基跨环ox-Michael加成-取代的四氢吡喃环。
更新日期:2024-02-29
中文翻译:

大环化/跨环吡喃环化策略全合成 (−)-Enigmodium A
本文公开了(-)-恩尼格唑A的18步合成。本合成基于三个具有相似复杂性的构件的模块化组装、用于形成18元大环骨架的大环闭环复分解、以及用于立体选择性构建2,6-顺式的脱甲硅烷基跨环ox-Michael加成-取代的四氢吡喃环。































 京公网安备 11010802027423号
京公网安备 11010802027423号