当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formaldehyde-Mediated Hydride Liberation of Alkylamines for Intermolecular Reactions in Hexafluoroisopropanol
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-02-26 , DOI: 10.1021/jacs.3c12215 Shaokun Cai 1 , Hong Tang 1 , Bo Li 1 , Yingbo Shao 1 , Danqi Zhang 1 , Hanliang Zheng 1 , Tianjiao Qiao 1 , Xin Chu 1 , Gang He 1 , Xiao-Song Xue 2 , Gong Chen 1, 3, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-02-26 , DOI: 10.1021/jacs.3c12215 Shaokun Cai 1 , Hong Tang 1 , Bo Li 1 , Yingbo Shao 1 , Danqi Zhang 1 , Hanliang Zheng 1 , Tianjiao Qiao 1 , Xin Chu 1 , Gang He 1 , Xiao-Song Xue 2 , Gong Chen 1, 3, 4
Affiliation
|
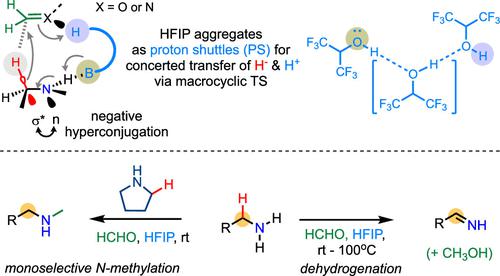
The ability of alkylamines to spontaneously liberate hydride ions is typically restrained, except under specific intramolecular reaction settings. Herein, we demonstrate that this reactivity can be unlocked through simple treatment with formaldehyde in hexafluoroisopropanol (HFIP) solvent, thereby enabling various intermolecular hydride transfer reactions of alkylamines under mild conditions. Besides transformations of small molecules, these reactions enable unique late-stage modification of complex peptides. Mechanistic investigations uncover that the key to these intermolecular hydride transfer processes lies in the accommodating conformation of solvent-mediated macrocyclic transition states, where the aggregates of HFIP molecules act as dexterous proton shuttles. Importantly, negative hyperconjugation between the lone electron pair of nitrogen and the antibonding orbital of amine’s α C–H bond plays a critical role in the C–H activation, promoting its hydride liberation.
中文翻译:

六氟异丙醇中分子间反应中甲醛介导的烷基胺氢化物释放
烷基胺自发释放氢阴离子的能力通常受到限制,除非在特定的分子内反应设置下。在此,我们证明这种反应活性可以通过在六氟异丙醇(HFIP)溶剂中用甲醛进行简单处理来释放,从而在温和条件下实现烷基胺的各种分子间氢化物转移反应。除了小分子的转化之外,这些反应还可以对复杂肽进行独特的后期修饰。机理研究发现,这些分子间氢化物转移过程的关键在于溶剂介导的大环过渡态的调节构象,其中 HFIP 分子的聚集体充当灵巧的质子穿梭机。重要的是,氮的孤电子对与胺的αC-H键的反键轨道之间的负超共轭在C-H活化中起着关键作用,促进其氢化物释放。
更新日期:2024-02-26
中文翻译:

六氟异丙醇中分子间反应中甲醛介导的烷基胺氢化物释放
烷基胺自发释放氢阴离子的能力通常受到限制,除非在特定的分子内反应设置下。在此,我们证明这种反应活性可以通过在六氟异丙醇(HFIP)溶剂中用甲醛进行简单处理来释放,从而在温和条件下实现烷基胺的各种分子间氢化物转移反应。除了小分子的转化之外,这些反应还可以对复杂肽进行独特的后期修饰。机理研究发现,这些分子间氢化物转移过程的关键在于溶剂介导的大环过渡态的调节构象,其中 HFIP 分子的聚集体充当灵巧的质子穿梭机。重要的是,氮的孤电子对与胺的αC-H键的反键轨道之间的负超共轭在C-H活化中起着关键作用,促进其氢化物释放。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号