当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Accessing Highly Substituted Indoles via B(C6F5)3-Catalyzed Secondary Alkyl Group Transfer
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-02-22 , DOI: 10.1021/acs.joc.4c00025
Salma A Elsherbeni 1, 2 , Rebecca L Melen 3 , Alexander P Pulis 4 , Louis C Morrill 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-02-22 , DOI: 10.1021/acs.joc.4c00025
Salma A Elsherbeni 1, 2 , Rebecca L Melen 3 , Alexander P Pulis 4 , Louis C Morrill 1
Affiliation
|
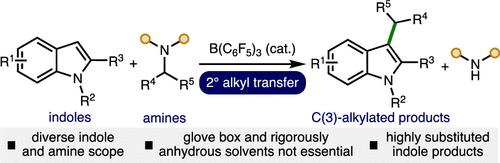
Herein, we report a synthetic method to access a range of highly substituted indoles via the B(C6F5)3-catalyzed transfer of 2° alkyl groups from amines. The transition-metal-free catalytic approach has been demonstrated across a broad range of indoles and amine 2° alkyl donors, including various substituents on both reacting components, to access useful C(3)-alkylated indole products. The alkyl transfer process can be performed using Schlenk line techniques in combination with commercially available B(C6F5)3·nH2O and solvents, which obviates the requirement for specialized equipment (e.g., glovebox).
中文翻译:

通过 B(C6F5)3 催化的仲烷基转移获得高度取代的吲哚
在此,我们报告了一种通过 B(C 6 F 5 ) 3催化的胺的 2° 烷基转移获得一系列高度取代的吲哚的合成方法。无过渡金属的催化方法已在广泛的吲哚和胺 2° 烷基供体(包括两种反应组分上的各种取代基)中得到证明,以获得有用的 C(3)-烷基化吲哚产物。烷基转移过程可以使用Schlenk线技术结合市售的B(C 6 F 5 ) 3 · n H 2 O和溶剂来进行,这消除了对专用设备(例如手套箱)的需要。
更新日期:2024-02-22
中文翻译:

通过 B(C6F5)3 催化的仲烷基转移获得高度取代的吲哚
在此,我们报告了一种通过 B(C 6 F 5 ) 3催化的胺的 2° 烷基转移获得一系列高度取代的吲哚的合成方法。无过渡金属的催化方法已在广泛的吲哚和胺 2° 烷基供体(包括两种反应组分上的各种取代基)中得到证明,以获得有用的 C(3)-烷基化吲哚产物。烷基转移过程可以使用Schlenk线技术结合市售的B(C 6 F 5 ) 3 · n H 2 O和溶剂来进行,这消除了对专用设备(例如手套箱)的需要。

































 京公网安备 11010802027423号
京公网安备 11010802027423号