当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
La(OTf)3-Catalyzed [3+2] Cycloaddition Reactions for the Synthesis of Benzo[d]oxazoles/Benzofurans
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-02-23 , DOI: 10.1021/acs.joc.3c02641 Zhanjun Li 1, 2 , Yalin Zhang 1 , Manman Sun 3 , Ye Zhang 1 , Zhaoxiang Lu 1 , Yupeng Deng 1 , Xianqiang Huang 1 , Guodong Shen 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-02-23 , DOI: 10.1021/acs.joc.3c02641 Zhanjun Li 1, 2 , Yalin Zhang 1 , Manman Sun 3 , Ye Zhang 1 , Zhaoxiang Lu 1 , Yupeng Deng 1 , Xianqiang Huang 1 , Guodong Shen 1
Affiliation
|
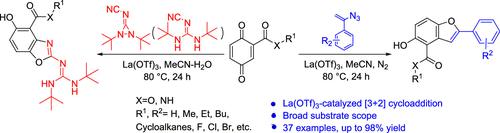
The La(OTf)3-catalyzed [3+2] cycloaddition reactions for the synthesis of benzo[d]oxazoles/benzofurans via quinones and 1,2-di-tert-butyl-3-(cyanimino)diaziridine (1,3-di-tert-butyl-2-cyanoguanidine)/vinyl azides have been explored. A series of 5-hydroxybenzofuran-4-carboxylic acid derivatives and 5-hydroxybenzo[d]oxazole-4-carboxylic acid derivatives were conveniently obtained with high yields and good stereoselectivities, which could be used for further transformations to valuable compounds.
中文翻译:

La(OTf)3-催化[3+2]环加成反应合成苯并[d]恶唑/苯并呋喃
La(OTf) 3催化的[3+2]环加成反应通过醌和1,2-二叔丁基-3-(氰胺基)二氮丙啶合成苯并[ d ]恶唑/苯并呋喃(1,3-二叔丁基-2-氰基胍)/乙烯基叠氮化物已被探索。方便地得到了一系列5-羟基苯并呋喃-4-甲酸衍生物和5-羟基苯并[ d ]恶唑-4-甲酸衍生物,收率高,立体选择性好,可用于进一步转化为有价值的化合物。
更新日期:2024-02-23
中文翻译:

La(OTf)3-催化[3+2]环加成反应合成苯并[d]恶唑/苯并呋喃
La(OTf) 3催化的[3+2]环加成反应通过醌和1,2-二叔丁基-3-(氰胺基)二氮丙啶合成苯并[ d ]恶唑/苯并呋喃(1,3-二叔丁基-2-氰基胍)/乙烯基叠氮化物已被探索。方便地得到了一系列5-羟基苯并呋喃-4-甲酸衍生物和5-羟基苯并[ d ]恶唑-4-甲酸衍生物,收率高,立体选择性好,可用于进一步转化为有价值的化合物。































 京公网安备 11010802027423号
京公网安备 11010802027423号