Synthesis ( IF 2.2 ) Pub Date : 2024-02-22 , DOI: 10.1055/a-2260-5652 Shinya Harusawa , Hiroki Yoneyama , Yoshihide Usami
|
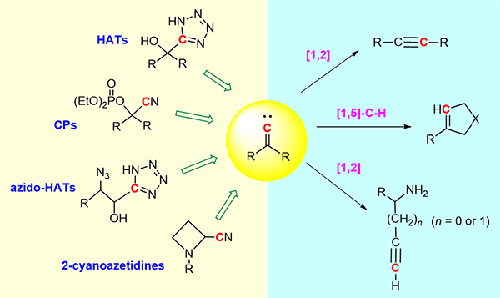
Tetrazole fragmentation under mild conditions from 5-hydroxyalkyl-1H-tetrazoles (HATs) or cyanophosphates (CPs) readily generates the corresponding alkylidene carbenes, which undergo [1,2]-rearrangements or [1,5]-C–H insertions to yield one-carbon homologous alkynes or five-membered unsaturated carbocycles, respectively. Furthermore, azido-HATs and 2-cyanoazetidines afford propargylic and homopropargylic amines, respectively, via [1,2]-rearrangement of the corresponding aminoalkyl alkylidene carbenes. These reactions are successfully applied for the synthesis of various biofunctional molecules. This short review summarizes the progress made on this methodology over the last decade.
1 Introduction
2 Early Studies
3 Tetrazole Fragmentation of HATs, Azido-HATs, and 2-Cyanoazetidines
4 HAT Synthetic Methods
5 Tetrazole Fragmentation from CPs
6 Summary and Perspective
中文翻译:

通过四唑裂解生成亚烷基卡宾:有机合成的最新进展和应用
在温和条件下,5-羟烷基-1H-四唑 (HAT) 或氰磷酸酯 (CP) 的四唑裂解很容易生成相应的亚烷基卡宾,这些卡宾经过 [1,2]-重排或 [1,5]-C-H 插入,生成分别产生一碳同系炔或五元不饱和碳环。此外,叠氮基-HAT和2-氰基氮杂环丁烷通过相应的氨基烷基亚烷基卡宾的[1,2]-重排分别提供炔丙胺和均丙炔胺。这些反应已成功应用于各种生物功能分子的合成。这篇简短的回顾总结了过去十年中这种方法所取得的进展。
1 简介
2 早期研究
3 HAT、叠氮-HAT 和 2-氰基氮杂环丁烷的四唑断裂
4 HAT合成方法
5 CP 中的四唑断裂
6 总结与展望


















































 京公网安备 11010802027423号
京公网安备 11010802027423号