Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revisiting catalytic His and Glu residues in coproporphyrin ferrochelatase – unexpected activities of active site variants
The FEBS Journal ( IF 5.5 ) Pub Date : 2024-02-23 , DOI: 10.1111/febs.17101 Thomas Gabler 1 , Andrea Dali 2 , Marzia Bellei 3 , Federico Sebastiani 2 , Maurizio Becucci 2 , Gianantonio Battistuzzi 4 , Paul Georg Furtmüller 1 , Giulietta Smulevich 2, 5 , Stefan Hofbauer 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2024-02-23 , DOI: 10.1111/febs.17101 Thomas Gabler 1 , Andrea Dali 2 , Marzia Bellei 3 , Federico Sebastiani 2 , Maurizio Becucci 2 , Gianantonio Battistuzzi 4 , Paul Georg Furtmüller 1 , Giulietta Smulevich 2, 5 , Stefan Hofbauer 1
Affiliation
|
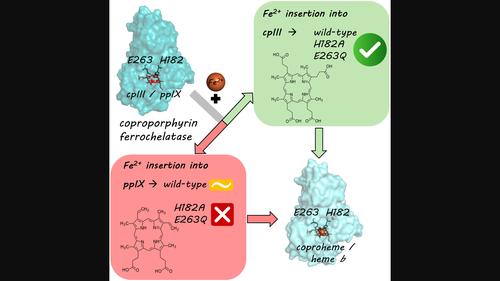
The identification of the coproporphyrin‐dependent heme biosynthetic pathway, which is used almost exclusively by monoderm bacteria in 2015 by Dailey et al . triggered studies aimed at investigating the enzymes involved in this pathway that were originally assigned to the protoporphyrin‐dependent heme biosynthetic pathway. Here, we revisit the active site of coproporphyrin ferrochelatase by a biophysical and biochemical investigation using the physiological substrate coproporphyrin III, which in contrast to the previously used substrate protoporphyrin IX has four propionate substituents and no vinyl groups. In particular, we have compared the reactivity of wild‐type coproporphyrin ferrochelatase from the firmicute Listeria monocytogenes with those of variants, namely, His182Ala (H182A) and Glu263Gln (E263Q), involving two key active site residues. Interestingly, both variants are active only toward the physiological substrate coproporphyrin III but inactive toward protoporphyrin IX. In addition, E263 exchange impairs the final oxidation step from ferrous coproheme to ferric coproheme. The characteristics of the active site in the context of the residues involved and the substrate binding properties are discussed here using structural and functional means, providing a further contribution to the deciphering of this enigmatic reaction mechanism.
中文翻译:

重新审视粪卟啉亚铁螯合酶中的催化 His 和 Glu 残基——活性位点变体的意外活性
Dailey 于 2015 年鉴定了粪卟啉依赖性血红素生物合成途径,该途径几乎完全由单皮细菌使用等人。引发了旨在调查该途径中涉及的酶的研究,这些酶最初被分配给原卟啉依赖性血红素生物合成途径。在这里,我们使用生理底物粪卟啉 III 通过生物物理和生化研究重新审视粪卟啉亚铁螯合酶的活性位点,与之前使用的底物原卟啉 IX 相比,它具有四个丙酸酯取代基且没有乙烯基。特别是,我们比较了来自厚壁菌门的野生型粪卟啉亚铁螯合酶的反应性单核细胞增生李斯特氏菌与变体的那些,即His182Ala (H182A)和Glu263Gln (E263Q),涉及两个关键活性位点残基。有趣的是,这两种变体仅对生理底物粪卟啉 III 有活性,但对原卟啉 IX 无活性。此外,E263 交换损害了从亚铁粪血红素到铁粪血红素的最终氧化步骤。这里使用结构和功能手段讨论了所涉及残基的活性位点特征和底物结合特性,为破译这种神秘的反应机制提供了进一步的贡献。
更新日期:2024-02-23
中文翻译:

重新审视粪卟啉亚铁螯合酶中的催化 His 和 Glu 残基——活性位点变体的意外活性
Dailey 于 2015 年鉴定了粪卟啉依赖性血红素生物合成途径,该途径几乎完全由单皮细菌使用等人。引发了旨在调查该途径中涉及的酶的研究,这些酶最初被分配给原卟啉依赖性血红素生物合成途径。在这里,我们使用生理底物粪卟啉 III 通过生物物理和生化研究重新审视粪卟啉亚铁螯合酶的活性位点,与之前使用的底物原卟啉 IX 相比,它具有四个丙酸酯取代基且没有乙烯基。特别是,我们比较了来自厚壁菌门的野生型粪卟啉亚铁螯合酶的反应性单核细胞增生李斯特氏菌与变体的那些,即His182Ala (H182A)和Glu263Gln (E263Q),涉及两个关键活性位点残基。有趣的是,这两种变体仅对生理底物粪卟啉 III 有活性,但对原卟啉 IX 无活性。此外,E263 交换损害了从亚铁粪血红素到铁粪血红素的最终氧化步骤。这里使用结构和功能手段讨论了所涉及残基的活性位点特征和底物结合特性,为破译这种神秘的反应机制提供了进一步的贡献。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号