当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical study on the kinetics and reaction mechanism involved in the reduction of quinone by 1-benzyl-1,4-dihydronicotinamide
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2024-02-22 , DOI: 10.1002/poc.4605 Manuel E. Medina 1 , Hugo A. Jiménez‐Vazquez 2 , Luis G. Zepeda‐Vallejo 1 , Ángel Trigos 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2024-02-22 , DOI: 10.1002/poc.4605 Manuel E. Medina 1 , Hugo A. Jiménez‐Vazquez 2 , Luis G. Zepeda‐Vallejo 1 , Ángel Trigos 1
Affiliation
|
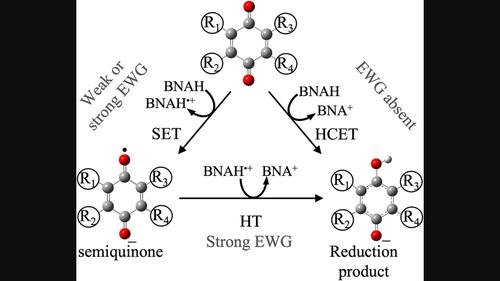
Although it is well known that coenzyme NAD(P)H is involved in anabolic and catabolic reactions in the living organism, there is still significant controversy over the reaction mechanism involved in this biochemical transformation. Thus, 1-benzyl-1,4-dihydronicotinamide was used as a NAD(P)H model in the reduction reaction of 1,4-benzoquinone (Q), 2,3,5,6-tetrachloro-1,4-benzoquinone, and 2,3-dicyano-1,4-benzoquinone in acetonitrile medium. The kinetic calculations support that formal hydride transfer is the main mechanism promoting Q reduction, while the two-step process dominates 2,3-dicyano-1,4-benzoquinone reduction. Interestingly, only the single-electron transfer mechanism takes place when 2,3,5,6-tetrachloro-1,4-benzoquinone is used, affording the corresponding semiquinone derivative as the main product. This mechanistic behavior is related to the presence or absence of electron-withdrawing groups in the quinones used. Furthermore, the kinetic study results showed that calculated reaction rate constants are in close agreement with experimental results. The results support that formal hydride transfer on the reduction reaction of Q by 1-benzyl-1,4-dihydronicotinamide in acetonitrile proceeds through a hydrogen coupled electron transfer mechanism. This theoretical analysis provides valuable knowledge that can be extrapolated to study the reduction of quinones performed by NADH and NADPH in physiological media.
中文翻译:

1-苄基-1,4-二氢烟酰胺还原醌的动力学及反应机理理论研究
尽管众所周知辅酶NAD(P)H参与生物体的合成代谢和分解代谢反应,但对于这种生化转化所涉及的反应机制仍存在重大争议。因此,1-苄基-1,4-二氢烟酰胺在1,4-苯醌(Q)、2,3,5,6-四氯-1,4-苯醌的还原反应中用作NAD(P)H模型和 2,3-二氰基-1,4-苯醌在乙腈介质中。动力学计算支持形式氢化物转移是促进Q还原的主要机制,而两步过程主导2,3-二氰基-1,4-苯醌还原。有趣的是,当使用2,3,5,6-四氯-1,4-苯醌时,仅发生单电子转移机制,得到相应的半醌衍生物作为主要产物。这种机械行为与所用醌中是否存在吸电子基团有关。此外,动力学研究结果表明,计算的反应速率常数与实验结果非常吻合。结果表明,乙腈中 1-苄基-1,4-二氢烟酰胺对 Q 的还原反应中的形式氢化物转移是通过氢偶联电子转移机制进行的。该理论分析提供了宝贵的知识,可以推断这些知识用于研究生理介质中 NADH 和 NADPH 进行的醌还原作用。
更新日期:2024-02-22
中文翻译:

1-苄基-1,4-二氢烟酰胺还原醌的动力学及反应机理理论研究
尽管众所周知辅酶NAD(P)H参与生物体的合成代谢和分解代谢反应,但对于这种生化转化所涉及的反应机制仍存在重大争议。因此,1-苄基-1,4-二氢烟酰胺在1,4-苯醌(Q)、2,3,5,6-四氯-1,4-苯醌的还原反应中用作NAD(P)H模型和 2,3-二氰基-1,4-苯醌在乙腈介质中。动力学计算支持形式氢化物转移是促进Q还原的主要机制,而两步过程主导2,3-二氰基-1,4-苯醌还原。有趣的是,当使用2,3,5,6-四氯-1,4-苯醌时,仅发生单电子转移机制,得到相应的半醌衍生物作为主要产物。这种机械行为与所用醌中是否存在吸电子基团有关。此外,动力学研究结果表明,计算的反应速率常数与实验结果非常吻合。结果表明,乙腈中 1-苄基-1,4-二氢烟酰胺对 Q 的还原反应中的形式氢化物转移是通过氢偶联电子转移机制进行的。该理论分析提供了宝贵的知识,可以推断这些知识用于研究生理介质中 NADH 和 NADPH 进行的醌还原作用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号