当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction Paradigms that Leverage Cycloaddition and Ring Strain to Construction Bicyclic Aryl Bioisosteres from Bicyclo[1.1.0]butanes
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2024-02-21 , DOI: 10.1002/ajoc.202400045 Stephen J. Sujansky 1 , Xiaoshen Ma 2
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2024-02-21 , DOI: 10.1002/ajoc.202400045 Stephen J. Sujansky 1 , Xiaoshen Ma 2
Affiliation
|
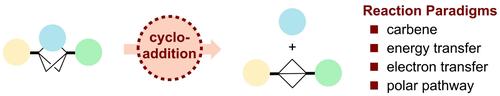
Within a medicinal chemist's toolbox, one of the most effective strategies to improve the overall properties of a biologically active compound is bioisosteric replacement. Ever since the first example of replacing benzene with a bicyclo[1.1.1]pentane (BCP) group was published in the late 1990s,[1] the medicinal chemistry community has continually been expanding the scope of such phenyl bioisosteric replacements. Recent interest from academia has focused on novel synthetic strategies to access C(sp3)-rich bicyclic hydrocarbons with expanded ring sizes. Herein, we summarize some of these transformations and reveal that most rely on strain releasing cycloadditions with bicyclo[1.1.0]butane (BCB) and bicyclo[2.1.0]pentane (housane). We have organized this review based on the mechanism of such strain release strategies, namely, carbene cycloadditions, energy transfer photocatalyzed cycloadditions, electron transfer catalyzed cycloadditions, and polar cycloadditions.
中文翻译:

利用环加成和环应变从双环[1.1.0]丁烷构建双环芳基生物等排体的反应范例
在药物化学家的工具箱中,改善生物活性化合物整体特性的最有效策略之一是生物等排替代。自从 20 世纪 90 年代末发表第一个用双环[1.1.1]戊烷 (BCP) 基团取代苯的例子以来,[1]药物化学界一直在不断扩大此类苯基生物等排取代的范围。最近学术界的兴趣集中在新的合成策略上,以获取具有扩大环尺寸的富含 C( sp 3 ) 的双环烃。在此,我们总结了其中一些转化,并揭示大多数依赖于双环[1.1.0]丁烷(BCB)和双环[2.1.0]戊烷(housane)的应变释放环加成。我们根据此类应变释放策略的机理组织了这篇综述,即卡宾环加成、能量转移光催化环加成、电子转移催化环加成和极性环加成。
更新日期:2024-02-21
中文翻译:

利用环加成和环应变从双环[1.1.0]丁烷构建双环芳基生物等排体的反应范例
在药物化学家的工具箱中,改善生物活性化合物整体特性的最有效策略之一是生物等排替代。自从 20 世纪 90 年代末发表第一个用双环[1.1.1]戊烷 (BCP) 基团取代苯的例子以来,[1]药物化学界一直在不断扩大此类苯基生物等排取代的范围。最近学术界的兴趣集中在新的合成策略上,以获取具有扩大环尺寸的富含 C( sp 3 ) 的双环烃。在此,我们总结了其中一些转化,并揭示大多数依赖于双环[1.1.0]丁烷(BCB)和双环[2.1.0]戊烷(housane)的应变释放环加成。我们根据此类应变释放策略的机理组织了这篇综述,即卡宾环加成、能量转移光催化环加成、电子转移催化环加成和极性环加成。

































 京公网安备 11010802027423号
京公网安备 11010802027423号