当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Weakened Interfacial Hydrogen Bond Connectivity Drives Selective Photocatalytic Water Oxidation toward H2O2 at Water/Brookite-TiO2 Interface
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-02-22 , DOI: 10.1021/jacs.3c13402
Guanhua Ren 1 , Min Zhou 1 , Haifeng Wang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-02-22 , DOI: 10.1021/jacs.3c13402
Guanhua Ren 1 , Min Zhou 1 , Haifeng Wang 1
Affiliation
|
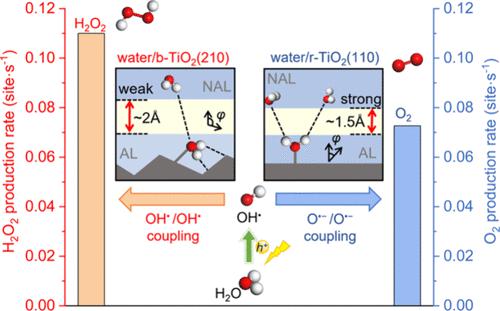
The formation of H2O2 through the two-electron photocatalytic water oxidation reaction (WOR) is significant but encounters the competition with the four-electron O2 evolution reaction. Recent studies showed a crystal-phase dependence in H2O2 selectivity, where high purity brookite TiO2 (b-TiO2) exhibits remarkable H2O2 selectivity in contrast to the common rutile phase TiO2 (r-TiO2). However, the origin of such a structure-induced selectivity preference remains elusive, primarily due to the complexities associated with the solid–liquid interface system and excited-state chemistry. Herein, we conducted a comprehensive investigation into the selectivity mechanism of WOR at the water/b-TiO2(210) and water/r-TiO2(110) interfaces, employing first-principles molecular dynamics simulations and microkinetic analyses. Intriguingly, our results reveal that the intrinsic catalytic ability of the b-TiO2(210) itself does not enhance H2O2 selectivity compared to r-TiO2(110). Instead, it is the weakened interfacial hydrogen bond connectivity, modulated by the herringbone-like local atomic structure of the b-TiO2(210) surface, that determines the selectivity. Specifically, this weakened H-bond connectivity (i.e., local low water density) at the interface, owing to the strong water adsorption and distinct adsorption orientation, can stabilize the OH• radical and inhibit its deprotonation, leading to an improved H2O2 selectivity. By contrast, the relatively strong interface H-bond connectivity established over r-TiO2(110) accelerates the deprotonation of OH•, with the OH• coverage being 3 orders of magnitude lower than at the water/b-TiO2(210) interface. This study quantitatively demonstrates that the local H-bond structure (water density) at the liquid/solid interface significantly influences photocatalytic selectivity, and this insight may offer a rational approach to enhance the H2O2 selectivity.
中文翻译:

界面氢键连通性减弱导致水/板钛矿-TiO2 界面处选择性光催化水氧化为 H2O2
通过双电子光催化水氧化反应(WOR)形成H 2 O 2很重要,但遇到了与四电子O 2析出反应的竞争。最近的研究表明,H 2 O 2选择性与晶相相关,其中高纯度板钛矿TiO 2 (b-TiO 2 ) 与常见的金红石相TiO 2 (r-TiO 2 ) 相比表现出显着的H 2 O 2选择性。然而,这种结构诱导的选择性偏好的起源仍然难以捉摸,主要是由于与固液界面系统和激发态化学相关的复杂性。在此,我们采用第一原理分子动力学模拟和微动力学分析,对水/b-TiO 2 (210) 和水/r-TiO 2 (110) 界面上的WOR选择性机制进行了全面研究。有趣的是,我们的结果表明,与 r-TiO 2 (110) 相比,b-TiO 2 (210) 本身的固有催化能力并没有增强 H 2 O 2选择性。相反,由 b-TiO 2 (210) 表面的人字形局部原子结构调节的减弱的界面氢键连接性决定了选择性。具体来说,这种弱化的氢键连接性(即界面处由于水的强烈吸附和明显的吸附方向,可以稳定OH ·自由基并抑制其去质子化,从而提高H 2 O 2的选择性。相比之下,r-TiO 2 (110) 上建立的相对较强的界面氢键连接加速了 OH •的去质子化,OH •覆盖率比水/b-TiO 2 (210) 低 3 个数量级界面。这项研究定量地表明,液/固界面的局部氢键结构(水密度)显着影响光催化选择性,这一见解可能为提高H 2 O 2选择性提供合理的方法。
更新日期:2024-02-22
中文翻译:

界面氢键连通性减弱导致水/板钛矿-TiO2 界面处选择性光催化水氧化为 H2O2
通过双电子光催化水氧化反应(WOR)形成H 2 O 2很重要,但遇到了与四电子O 2析出反应的竞争。最近的研究表明,H 2 O 2选择性与晶相相关,其中高纯度板钛矿TiO 2 (b-TiO 2 ) 与常见的金红石相TiO 2 (r-TiO 2 ) 相比表现出显着的H 2 O 2选择性。然而,这种结构诱导的选择性偏好的起源仍然难以捉摸,主要是由于与固液界面系统和激发态化学相关的复杂性。在此,我们采用第一原理分子动力学模拟和微动力学分析,对水/b-TiO 2 (210) 和水/r-TiO 2 (110) 界面上的WOR选择性机制进行了全面研究。有趣的是,我们的结果表明,与 r-TiO 2 (110) 相比,b-TiO 2 (210) 本身的固有催化能力并没有增强 H 2 O 2选择性。相反,由 b-TiO 2 (210) 表面的人字形局部原子结构调节的减弱的界面氢键连接性决定了选择性。具体来说,这种弱化的氢键连接性(即界面处由于水的强烈吸附和明显的吸附方向,可以稳定OH ·自由基并抑制其去质子化,从而提高H 2 O 2的选择性。相比之下,r-TiO 2 (110) 上建立的相对较强的界面氢键连接加速了 OH •的去质子化,OH •覆盖率比水/b-TiO 2 (210) 低 3 个数量级界面。这项研究定量地表明,液/固界面的局部氢键结构(水密度)显着影响光催化选择性,这一见解可能为提高H 2 O 2选择性提供合理的方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号