当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Avenue to novel o-carboranyl boron compounds – reactivity study of o-carborane-fused aminoborirane towards organic azides
Chemical Science ( IF 7.6 ) Pub Date : 2024-02-22 , DOI: 10.1039/d4sc00489b
Junyi Wang 1, 2 , Libo Xiang 3, 4 , Xiaocui Liu 1 , Alexander Matler 3, 4 , Zhenyang Lin 2 , Qing Ye 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2024-02-22 , DOI: 10.1039/d4sc00489b
Junyi Wang 1, 2 , Libo Xiang 3, 4 , Xiaocui Liu 1 , Alexander Matler 3, 4 , Zhenyang Lin 2 , Qing Ye 3, 4
Affiliation
|
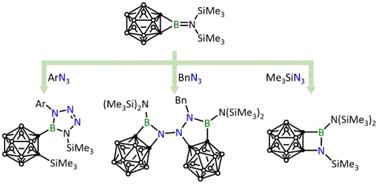
Herein we report the reactivity study of o-carborane-fused bis(trimethylsilyl)aminoborirane towards three different types of organic azides, i.e., aryl, alkyl, and silyl azides. The reaction with ArN3 (Ar = 2,6-iPr2C6H4, 2,6-C6H3Cl2, 2,4,6-C6H2Br3, C6F5) resulted in the cycloaddition of ArN3 to the borirane BN unit accompanied by silyl migration. Conversely, in the reaction with BnN3, only the BnN3 : borirane 1 : 2 ring expansion product was obtained. Finally, the reaction with Me3SiN3 resulted in a formal nitrene insertion product under thermal conditions. All of the newly obtained o-carborane-fused BN-containing heterocycles were fully characterized, and the mechanism of these substituent-dependent reactions was studied using DFT calculations.
中文翻译:

新型邻碳硼基硼化合物之路——邻碳硼烷稠合氨基硼烷对有机叠氮化物的反应性研究
在此,我们报道了邻碳硼烷稠合双(三甲基甲硅烷基)氨基硼烷对三种不同类型的有机叠氮化物(即芳基、烷基和甲硅烷基叠氮化物)的反应性研究。与ArN 3 (Ar = 2,6-iPr 2 C 6 H 4 , 2,6-C 6 H 3 Cl 2 , 2,4,6-C 6 H 2 Br 3 , C 6 F 5 ) 的反应产生ArN 3环加成到硼烷BN单元上并伴随着甲硅烷基迁移。相反,在与BnN 3的反应中,仅获得BnN 3 :硼烷1:2扩环产物。最后,与Me 3 SiN 3的反应在热条件下产生正式的氮烯插入产物。所有新获得的邻碳硼烷稠合的含BN杂环都得到了充分的表征,并使用DFT计算研究了这些取代基依赖性反应的机理。
更新日期:2024-02-22
中文翻译:

新型邻碳硼基硼化合物之路——邻碳硼烷稠合氨基硼烷对有机叠氮化物的反应性研究
在此,我们报道了邻碳硼烷稠合双(三甲基甲硅烷基)氨基硼烷对三种不同类型的有机叠氮化物(即芳基、烷基和甲硅烷基叠氮化物)的反应性研究。与ArN 3 (Ar = 2,6-iPr 2 C 6 H 4 , 2,6-C 6 H 3 Cl 2 , 2,4,6-C 6 H 2 Br 3 , C 6 F 5 ) 的反应产生ArN 3环加成到硼烷BN单元上并伴随着甲硅烷基迁移。相反,在与BnN 3的反应中,仅获得BnN 3 :硼烷1:2扩环产物。最后,与Me 3 SiN 3的反应在热条件下产生正式的氮烯插入产物。所有新获得的邻碳硼烷稠合的含BN杂环都得到了充分的表征,并使用DFT计算研究了这些取代基依赖性反应的机理。

































 京公网安备 11010802027423号
京公网安备 11010802027423号