Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
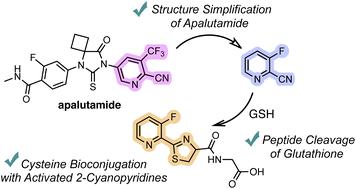
2-cyanopyridine derivatives enable N-terminal cysteine bioconjugation and peptide bond cleavage of glutathione under aqueous and mild conditions
RSC Advances ( IF 3.9 ) Pub Date : 2024-02-22 , DOI: 10.1039/d4ra00437j Tetsuya Yano 1 , Takahiro Yamada 1 , Hiroaki Isida 1 , Nami Ohashi 1 , Toshimasa Itoh 1
RSC Advances ( IF 3.9 ) Pub Date : 2024-02-22 , DOI: 10.1039/d4ra00437j Tetsuya Yano 1 , Takahiro Yamada 1 , Hiroaki Isida 1 , Nami Ohashi 1 , Toshimasa Itoh 1
Affiliation
|
Inspired by the chemical reactivity of apalutamide, we have developed an efficient method for N-terminal cysteine bioconjugation with 2-cyanopyridine derivatives. Systematic investigations of various 2-cyanopyridines revealed that 2-cyanopyridines with electron-withdrawing groups react efficiently with cysteine under aqueous and mild conditions. Moreover, the highly reactive 2-cyanopyridines enable the peptide bond cleavage of glutathione. The utility of our method is demonstrated by its application to the cysteine-selective chemical modification of bioactive peptides.
中文翻译:

2-氰基吡啶衍生物能够在水性和温和条件下实现 N 端半胱氨酸生物缀合和谷胱甘肽肽键裂解
受 apalutamide 化学反应性的启发,我们开发了一种有效的 N 端半胱氨酸与 2-氰基吡啶衍生物生物缀合方法。对各种2-氰基吡啶的系统研究表明,具有吸电子基团的2-氰基吡啶在水性和温和条件下可与半胱氨酸有效反应。此外,高反应性的 2-氰基吡啶能够裂解谷胱甘肽的肽键。我们的方法的实用性通过其在生物活性肽的半胱氨酸选择性化学修饰中的应用得到证明。
更新日期:2024-02-22
中文翻译:

2-氰基吡啶衍生物能够在水性和温和条件下实现 N 端半胱氨酸生物缀合和谷胱甘肽肽键裂解
受 apalutamide 化学反应性的启发,我们开发了一种有效的 N 端半胱氨酸与 2-氰基吡啶衍生物生物缀合方法。对各种2-氰基吡啶的系统研究表明,具有吸电子基团的2-氰基吡啶在水性和温和条件下可与半胱氨酸有效反应。此外,高反应性的 2-氰基吡啶能够裂解谷胱甘肽的肽键。我们的方法的实用性通过其在生物活性肽的半胱氨酸选择性化学修饰中的应用得到证明。































 京公网安备 11010802027423号
京公网安备 11010802027423号