当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of 3-dibutylamino-propylamine aqueous solution for CO2 capture: Promoting the energy-saving regeneration through self-extraction regulation
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-02-10 , DOI: 10.1016/j.cej.2024.149567 Zhangfeng Dong , Lijie Que , Wenjun Li , Qiuyao Ren , Chen Wang , Shixuan Li , Bihong Lv , Guohua Jing , Huazhen Shen
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-02-10 , DOI: 10.1016/j.cej.2024.149567 Zhangfeng Dong , Lijie Que , Wenjun Li , Qiuyao Ren , Chen Wang , Shixuan Li , Bihong Lv , Guohua Jing , Huazhen Shen
|
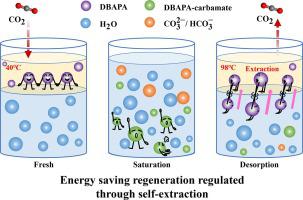
Due to the strong bonding of amino groups with CO2 , the alcohol amine-based absorbents for CO2 capture suffer the disadvantage of high regeneration temperature and energy consumption. To promote the energy-saving regeneration, a novel 3-Dibutylamino-propylamine (DBAPA) aqueous absorbent featured ‘self-extraction’ property was proposed, for which the fresh solution maintained liquid–liquid two-phase and became homogeneous after CO2 absorption. Due to the self-extraction regulation, its CO2 products could be regenerated at a low temperature below the boiling point of water (98 °C). It kept 81.98 % of its initial loading (1.27 mol·mol−1 ) after the first regeneration and maintained 73.17 % after the sixth regeneration cycle at 98 °C, much higher than that of MEA (28.96 %). Based on the results of 13 C NMR and quantum chemical calculations, the self-extraction regulation mechanism of DBAPA for CO2 capture was clarified. It was proved that there was a vast polarity difference between DBAPA and H2 O, resulting in phase separation before absorption. With the increasing CO2 loading, CO2 reacted with DBAPA to form DBAPA-carbamate, and the solution became homogeneous because of the increasing polarity of the CO2 products and the strong hydrogen bonds between the products and H2 O. The amine recovered to its low polarity during the desorption process, and the intermolecular hydrogen bonding with H2 O decreased. DBAPA separated from the main solution to the upper phase, promoting the regeneration rate at 98 °C. Based on this self-extraction property, the regeneration energy consumption of DBAPA was estimated to be 1.89 GJ·ton−1 CO2 , which was much lower than that of MEA (3.77 GJ·ton−1 CO2 ). The results indicated that the novel DBAPA aqueous absorbent with self-extraction characteristics had good energy-saving potential for CO2 capture.
中文翻译:

3-二丁基氨基丙胺水溶液捕集CO2的评价:通过自萃取调节促进节能再生
由于氨基与CO2的结合力很强,用于CO2捕获的醇胺基吸收剂存在再生温度高和能耗高的缺点。为了促进节能再生,提出了一种具有“自萃取”特性的新型3-二丁基氨基丙胺(DBAPA)水吸收剂,其新鲜溶液在吸收CO2后保持液-液两相并变得均匀。由于自萃取调节,其CO2产物可以在低于水沸点(98℃)的低温下再生。第一次再生后,其负载量保持在初始负载量的81.98%(1.27 mol·mol−1),在98℃的第六次再生循环后,负载量保持在73.17%,远高于MEA(28.96%)。基于13C NMR和量子化学计算的结果,阐明了DBAPA捕获CO2的自萃取调控机制。事实证明,DBAPA与H2O之间存在巨大的极性差异,导致吸收前发生相分离。随着CO2负载量的增加,CO2与DBAPA反应形成DBAPA-氨基甲酸酯,并且由于CO2产物的极性增加以及产物与H2O之间的强氢键,溶液变得均质。胺在解吸过程中恢复到低极性,与H2O的分子间氢键减少。 DBAPA从主溶液中分离到上相,促进了98℃下的再生速率。基于这种自萃取特性,DBAPA的再生能耗估计为1.89 GJ·ton−1 CO2,远低于MEA(3.77 GJ·ton−1 CO2)。 结果表明,具有自萃取特性的新型DBAPA水吸收剂在CO2捕集方面具有良好的节能潜力。
更新日期:2024-02-10
中文翻译:

3-二丁基氨基丙胺水溶液捕集CO2的评价:通过自萃取调节促进节能再生
由于氨基与CO2的结合力很强,用于CO2捕获的醇胺基吸收剂存在再生温度高和能耗高的缺点。为了促进节能再生,提出了一种具有“自萃取”特性的新型3-二丁基氨基丙胺(DBAPA)水吸收剂,其新鲜溶液在吸收CO2后保持液-液两相并变得均匀。由于自萃取调节,其CO2产物可以在低于水沸点(98℃)的低温下再生。第一次再生后,其负载量保持在初始负载量的81.98%(1.27 mol·mol−1),在98℃的第六次再生循环后,负载量保持在73.17%,远高于MEA(28.96%)。基于13C NMR和量子化学计算的结果,阐明了DBAPA捕获CO2的自萃取调控机制。事实证明,DBAPA与H2O之间存在巨大的极性差异,导致吸收前发生相分离。随着CO2负载量的增加,CO2与DBAPA反应形成DBAPA-氨基甲酸酯,并且由于CO2产物的极性增加以及产物与H2O之间的强氢键,溶液变得均质。胺在解吸过程中恢复到低极性,与H2O的分子间氢键减少。 DBAPA从主溶液中分离到上相,促进了98℃下的再生速率。基于这种自萃取特性,DBAPA的再生能耗估计为1.89 GJ·ton−1 CO2,远低于MEA(3.77 GJ·ton−1 CO2)。 结果表明,具有自萃取特性的新型DBAPA水吸收剂在CO2捕集方面具有良好的节能潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号