当前位置:
X-MOL 学术
›
Biochem. Biophys. Res. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Olodaterol promotes thermogenesis in brown adipocytes via regulation of the β2-AR/cAMP/PKA signaling pathway
Biochemical and Biophysical Research Communications ( IF 2.5 ) Pub Date : 2024-02-15 , DOI: 10.1016/j.bbrc.2024.149689 Le Wang 1 , Zhaobin Lei 1 , Guanjie Zhang 1 , Yang Cheng 1 , Mingwei Zhong 1 , Guangyong Zhang 1 , Sanyuan Hu 1
Biochemical and Biophysical Research Communications ( IF 2.5 ) Pub Date : 2024-02-15 , DOI: 10.1016/j.bbrc.2024.149689 Le Wang 1 , Zhaobin Lei 1 , Guanjie Zhang 1 , Yang Cheng 1 , Mingwei Zhong 1 , Guangyong Zhang 1 , Sanyuan Hu 1
Affiliation
|
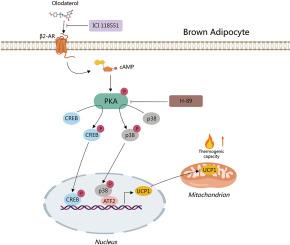
The escalating incidence of metabolic pathologies such as obesity and diabetes mellitus underscores the imperative for innovative therapeutics targeting lipid metabolism modulation. Within this context, augmenting thermogenic processes in adipose cells emerges as a viable therapeutic approach. Given the limitations of previous β3-adrenergic receptor (β3-AR) agonist treatments in human diseases, there is an increasing focus on therapies targeting the β2-adrenergic receptor (β2-AR). Olodaterol (OLO) is a potent β2-AR agonist that is a potential novel pharmacological candidate in this area. Our study explores the role and underlying mechanisms of OLO in enhancing brown adipose thermogenesis, providing robust evidence from in vitro and in vivo studies. OLO demonstrated a dose-dependent enhancement of lipolysis, notably increasing the expression of Uncoupling Protein 1 (UCP1) and raising the rate of oxygen consumption in primary brown adipocytes. This suggests a significant increase in thermogenic potential and energy expenditure. The administration of OLO to murine models noticeably enhanced cold-induced nonshivering thermogenesis. OLO elevated UCP1 expression in the brown adipose tissue of mice. Furthermore, it promoted brown adipocyte thermogenesis by activating the β2-AR/cAMP/PKA signaling cascades according to RNA sequencing, western blotting, and molecular docking analysis. This investigation underscores the therapeutic potential of OLO for metabolic ailments and sheds light on the intricate molecular dynamics of adipocyte thermogenesis, laying the groundwork for future targeted therapeutic interventions in human metabolic disorders.
中文翻译:

奥达特罗通过调节 β2-AR/cAMP/PKA 信号通路促进棕色脂肪细胞生热
肥胖和糖尿病等代谢病理学发病率的不断上升凸显了针对脂质代谢调节的创新疗法的必要性。在这种背景下,增强脂肪细胞的产热过程成为一种可行的治疗方法。鉴于先前β3-肾上腺素能受体(β3-AR)激动剂治疗人类疾病的局限性,人们越来越关注针对β2-肾上腺素能受体(β2-AR)的治疗。奥达特罗 (OLO) 是一种有效的 β2-AR 激动剂,是该领域潜在的新型药理学候选药物。我们的研究探讨了 OLO 在增强棕色脂肪生热作用中的作用和潜在机制,为体外和体内研究提供了强有力的证据。 OLO 表现出剂量依赖性的脂肪分解增强,特别是增加解偶联蛋白 1 (UCP1) 的表达并提高原代棕色脂肪细胞的耗氧率。这表明产热潜力和能量消耗显着增加。对小鼠模型施用 OLO 显着增强了寒冷诱导的非颤抖产热作用。 OLO 提高了小鼠棕色脂肪组织中 UCP1 的表达。此外,根据 RNA 测序、蛋白质印迹和分子对接分析,它通过激活 β2-AR/cAMP/PKA 信号级联来促进棕色脂肪细胞产热。这项研究强调了 OLO 对代谢疾病的治疗潜力,并揭示了脂肪细胞产热的复杂分子动力学,为未来人类代谢疾病的针对性治疗干预奠定了基础。
更新日期:2024-02-15
中文翻译:

奥达特罗通过调节 β2-AR/cAMP/PKA 信号通路促进棕色脂肪细胞生热
肥胖和糖尿病等代谢病理学发病率的不断上升凸显了针对脂质代谢调节的创新疗法的必要性。在这种背景下,增强脂肪细胞的产热过程成为一种可行的治疗方法。鉴于先前β3-肾上腺素能受体(β3-AR)激动剂治疗人类疾病的局限性,人们越来越关注针对β2-肾上腺素能受体(β2-AR)的治疗。奥达特罗 (OLO) 是一种有效的 β2-AR 激动剂,是该领域潜在的新型药理学候选药物。我们的研究探讨了 OLO 在增强棕色脂肪生热作用中的作用和潜在机制,为体外和体内研究提供了强有力的证据。 OLO 表现出剂量依赖性的脂肪分解增强,特别是增加解偶联蛋白 1 (UCP1) 的表达并提高原代棕色脂肪细胞的耗氧率。这表明产热潜力和能量消耗显着增加。对小鼠模型施用 OLO 显着增强了寒冷诱导的非颤抖产热作用。 OLO 提高了小鼠棕色脂肪组织中 UCP1 的表达。此外,根据 RNA 测序、蛋白质印迹和分子对接分析,它通过激活 β2-AR/cAMP/PKA 信号级联来促进棕色脂肪细胞产热。这项研究强调了 OLO 对代谢疾病的治疗潜力,并揭示了脂肪细胞产热的复杂分子动力学,为未来人类代谢疾病的针对性治疗干预奠定了基础。






























 京公网安备 11010802027423号
京公网安备 11010802027423号