当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sodium-Permeable Ion Channels TRPM4 and TRPM5 are Functional in Human Gastric Parietal Cells in Culture and Modulate the Cellular Response to Bitter-Tasting Food Constituents
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-02-20 , DOI: 10.1021/acs.jafc.3c09085
Phil Richter 1, 2 , Gaby Andersen 2 , Kristin Kahlenberg 2 , Alina Ulrike Mueller 2 , Philip Pirkwieser 2 , Valerie Boger 2 , Veronika Somoza 2, 3, 4
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-02-20 , DOI: 10.1021/acs.jafc.3c09085
Phil Richter 1, 2 , Gaby Andersen 2 , Kristin Kahlenberg 2 , Alina Ulrike Mueller 2 , Philip Pirkwieser 2 , Valerie Boger 2 , Veronika Somoza 2, 3, 4
Affiliation
|
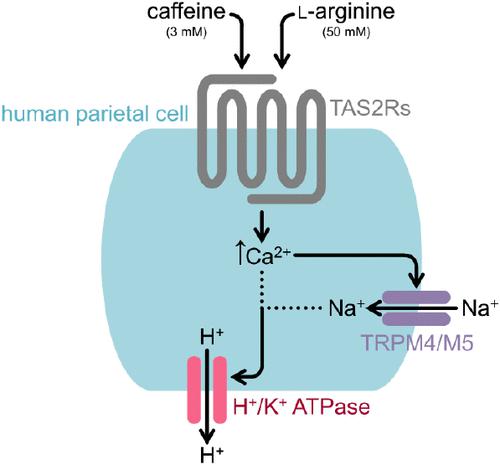
Gastric parietal cells secrete chloride ions and protons to form hydrochloric acid. Besides endogenous stimulants, e.g., acetylcholine, bitter-tasting food constituents, e.g., caffeine, induce proton secretion via interaction with bitter taste receptors (TAS2Rs), leading to increased cytosolic Ca2+ and cAMP concentrations. We hypothesized TAS2R activation by bitter tastants to result in proton secretion via cellular Na+ influx mediated by transient receptor potential channels (TRP) M4 and M5 in immortalized human parietal HGT-1 cells. Using the food-derived TAS2R agonists caffeine and l-arginine, we demonstrate both bitter compounds to induce a TRPM4/M5-mediated Na+ influx, with EC50 values of 0.65 and 10.38 mM, respectively, that stimulates cellular proton secretion. Functional involvement of TAS2Rs in the caffeine-evoked effect was demonstrated by means of the TAS2R antagonist homoeriodictyol, and stably CRISPR-Cas9-edited TAS2R43ko cells. Building on previous results, these data further support the suitability of HGT-1 cells as a surrogate cell model for taste cells. In addition, TRPM4/M5 mediated a Na+ influx after stimulating HGT-1 cells with the acetylcholine analogue carbachol, indicating an interaction of the digestion-associated cholinergic pathway with a taste-signaling pathway in parietal cells.
中文翻译:

钠渗透性离子通道 TRPM4 和 TRPM5 在培养的人胃壁细胞中发挥作用,并调节细胞对苦味食物成分的反应
胃壁细胞分泌氯离子和质子形成盐酸。除了内源性兴奋剂(例如乙酰胆碱)外,苦味食物成分(例如咖啡因)也可通过与苦味受体 (TAS2R) 相互作用诱导质子分泌,导致胞质 Ca 2+和 cAMP 浓度增加。我们假设苦味促味剂激活 TAS2R,导致永生化人壁层 HGT-1 细胞中瞬时受体电位通道 (TRP) M4 和 M5 介导的细胞 Na +流入导致质子分泌。使用食物来源的TAS2R激动剂咖啡因和l-精氨酸,我们证明这两种苦味化合物都能诱导TRPM4/M5介导的Na +流入,EC 50值分别为0.65和10.38 mM,从而刺激细胞质子分泌。通过 TAS2R 拮抗剂高圣草酚和稳定 CRISPR-Cas9 编辑的 TAS2R43ko 细胞证明了 TAS2R 在咖啡因诱发效应中的功能参与。基于之前的结果,这些数据进一步支持 HGT-1 细胞作为味觉细胞替代细胞模型的适用性。此外,TRPM4/M5 在用乙酰胆碱类似物卡巴胆碱刺激 HGT-1 细胞后介导 Na +流入,表明消化相关胆碱能途径与壁细胞中味觉信号途径的相互作用。
更新日期:2024-02-20
中文翻译:

钠渗透性离子通道 TRPM4 和 TRPM5 在培养的人胃壁细胞中发挥作用,并调节细胞对苦味食物成分的反应
胃壁细胞分泌氯离子和质子形成盐酸。除了内源性兴奋剂(例如乙酰胆碱)外,苦味食物成分(例如咖啡因)也可通过与苦味受体 (TAS2R) 相互作用诱导质子分泌,导致胞质 Ca 2+和 cAMP 浓度增加。我们假设苦味促味剂激活 TAS2R,导致永生化人壁层 HGT-1 细胞中瞬时受体电位通道 (TRP) M4 和 M5 介导的细胞 Na +流入导致质子分泌。使用食物来源的TAS2R激动剂咖啡因和l-精氨酸,我们证明这两种苦味化合物都能诱导TRPM4/M5介导的Na +流入,EC 50值分别为0.65和10.38 mM,从而刺激细胞质子分泌。通过 TAS2R 拮抗剂高圣草酚和稳定 CRISPR-Cas9 编辑的 TAS2R43ko 细胞证明了 TAS2R 在咖啡因诱发效应中的功能参与。基于之前的结果,这些数据进一步支持 HGT-1 细胞作为味觉细胞替代细胞模型的适用性。此外,TRPM4/M5 在用乙酰胆碱类似物卡巴胆碱刺激 HGT-1 细胞后介导 Na +流入,表明消化相关胆碱能途径与壁细胞中味觉信号途径的相互作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号