当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antibacterial Marinopyrroles and Pseudilins Act as Protonophores
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2024-02-20 , DOI: 10.1021/acschembio.3c00773
Gabriel Castro-Falcón 1 , Jan Straetener 2 , Jan Bornikoel 2 , Daniela Reimer 1 , Trevor N Purdy 1 , Anne Berscheid 2 , Florence M Schempp 1 , Dennis Y Liu 3 , Roger G Linington 3 , Heike Brötz-Oesterhelt 2, 4, 5 , Chambers C Hughes 1, 2, 4, 5
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2024-02-20 , DOI: 10.1021/acschembio.3c00773
Gabriel Castro-Falcón 1 , Jan Straetener 2 , Jan Bornikoel 2 , Daniela Reimer 1 , Trevor N Purdy 1 , Anne Berscheid 2 , Florence M Schempp 1 , Dennis Y Liu 3 , Roger G Linington 3 , Heike Brötz-Oesterhelt 2, 4, 5 , Chambers C Hughes 1, 2, 4, 5
Affiliation
|
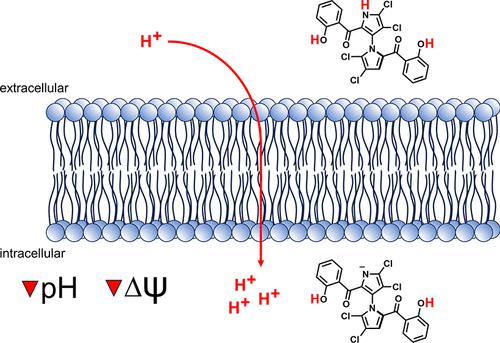
Elucidating the mechanism of action (MoA) of antibacterial natural products is crucial to evaluating their potential as novel antibiotics. Marinopyrroles, pentachloropseudilin, and pentabromopseudilin are densely halogenated, hybrid pyrrole-phenol natural products with potent activity against Gram-positive bacterial pathogens like Staphylococcus aureus. However, the exact way they exert this antibacterial activity has not been established. In this study, we explore their structure–activity relationship, determine their spatial location in bacterial cells, and investigate their MoA. We show that the natural products share a common MoA based on membrane depolarization and dissipation of the proton motive force (PMF) that is essential for cell viability. The compounds show potent protonophore activity but do not appear to destroy the integrity of the cytoplasmic membrane via the formation of larger pores or interfere with the stability of the peptidoglycan sacculus. Thus, our current model for the antibacterial MoA of marinopyrrole, pentachloropseudilin, and pentabromopseudilin stipulates that the acidic compounds insert into the membrane and transport protons inside the cell. This MoA may explain many of the deleterious biological effects in mammalian cells, plants, phytoplankton, viruses, and protozoans that have been reported for these compounds.
中文翻译:

抗菌 Marinopyrroles 和 Pseudilins 充当原载体
阐明抗菌天然产物的作用机制 (MoA) 对于评估其作为新型抗生素的潜力至关重要。马里诺吡咯、五氯杂二烯和五溴吡啶是密集卤化的杂化吡咯-苯酚天然产物,对金黄色葡萄球菌等革兰氏阳性细菌病原体具有强效活性。然而,它们发挥这种抗菌活性的确切方式尚未确定。在这项研究中,我们探讨了它们的构效关系,确定了它们在细菌细胞中的空间位置,并研究了它们的 MoA。我们表明,天然产物具有基于膜去极化和质子动力 (PMF) 耗散的共同 MoA,这对细胞活力至关重要。这些化合物显示出强大的原子细胞活性,但似乎不会通过形成更大的孔来破坏细胞质膜的完整性或干扰肽聚糖囊的稳定性。因此,我们目前的 marinopyrrole、pentachloropseudilin 和 pentabromopseudilin 的抗菌 MoA 模型规定酸性化合物插入细胞膜并在细胞内运输质子。该 MoA 可以解释已报道的这些化合物在哺乳动物细胞、植物、浮游植物、病毒和原生动物中的许多有害生物效应。
更新日期:2024-02-20
中文翻译:

抗菌 Marinopyrroles 和 Pseudilins 充当原载体
阐明抗菌天然产物的作用机制 (MoA) 对于评估其作为新型抗生素的潜力至关重要。马里诺吡咯、五氯杂二烯和五溴吡啶是密集卤化的杂化吡咯-苯酚天然产物,对金黄色葡萄球菌等革兰氏阳性细菌病原体具有强效活性。然而,它们发挥这种抗菌活性的确切方式尚未确定。在这项研究中,我们探讨了它们的构效关系,确定了它们在细菌细胞中的空间位置,并研究了它们的 MoA。我们表明,天然产物具有基于膜去极化和质子动力 (PMF) 耗散的共同 MoA,这对细胞活力至关重要。这些化合物显示出强大的原子细胞活性,但似乎不会通过形成更大的孔来破坏细胞质膜的完整性或干扰肽聚糖囊的稳定性。因此,我们目前的 marinopyrrole、pentachloropseudilin 和 pentabromopseudilin 的抗菌 MoA 模型规定酸性化合物插入细胞膜并在细胞内运输质子。该 MoA 可以解释已报道的这些化合物在哺乳动物细胞、植物、浮游植物、病毒和原生动物中的许多有害生物效应。

































 京公网安备 11010802027423号
京公网安备 11010802027423号