当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxygen Release and Incorporation Behaviors in BaFeO3 Polymorphs with Unusually High-Valence Fe4+
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-02-18 , DOI: 10.1021/acs.chemmater.3c03236
Rei Watanabe 1 , Masato Goto 1 , Yoshihisa Kosugi 1 , Daisuke Kan 1 , Yuichi Shimakawa 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2024-02-18 , DOI: 10.1021/acs.chemmater.3c03236
Rei Watanabe 1 , Masato Goto 1 , Yoshihisa Kosugi 1 , Daisuke Kan 1 , Yuichi Shimakawa 1
Affiliation
|
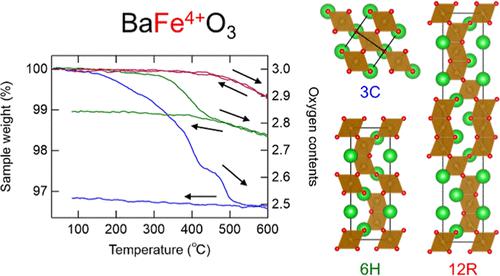
Fully oxygenated perovskite BaFeO3 containing unusually high-valence Fe4+ shows three crystal polymorphs with the same chemical composition. The 3C-type BaFeO3 has a simple cubic perovskite structure consisting of corner-sharing FeO6 octahedra, while the 6H- and 12R-type BaFeO3 have hexagonal perovskite structures consisting of both corner-sharing and face-sharing FeO6 octahedra. The compounds readily release oxygen into the air to reduce the high-valence state of the Fe ions, but the oxygen release behaviors strongly depend on the crystal structure. The 3C-type BaFeO3 releases oxygen topotactically from the corner-shared sites of the FeO6 octahedra at a temperature as low as 130 °C. In contrast, the 6H- and 12R-type BaFeO3 preferentially release oxygen from the face-shared sites above 320 and 460 °C, respectively, although they include the corner-shared sites in the crystal structures. The resultant oxygen-deficient 3C-type BaFeO2.5 does not incorporate back oxygen in air, whereas the 12R-type hexagonal structure shows completely reversible oxygen release and incorporation in air. Once the 12R-type structure is established, unusually high-valence states such as Fe4+ can be stabilized without extreme conditions.
中文翻译:

具有异常高价 Fe4+ 的 BaFeO3 多晶型物的氧释放和结合行为
含有异常高价Fe 4+的全氧化钙钛矿BaFeO 3显示出具有相同化学组成的三种晶体多晶型。3C型BaFeO 3具有由共角FeO 6八面体组成的简单立方钙钛矿结构,而6H型和12R型BaFeO 3具有由共角和共面FeO 6八面体组成的六方钙钛矿结构。这些化合物很容易将氧气释放到空气中以还原铁离子的高价态,但氧气释放行为很大程度上取决于晶体结构。3C型BaFeO 3在低至130℃的温度下从FeO 6八面体的角共享位点局部释放氧。相反,6H型和12R型BaFeO 3分别在320℃和460℃以上优先从面共享位释放氧,尽管它们在晶体结构中包括角共享位。所得缺氧的3C型BaFeO 2.5不会将空气中的氧带回,而12R型六方结构则显示出完全可逆的氧释放和空气中的结合。一旦建立了12R型结构,就可以在没有极端条件的情况下稳定异常高价态,例如Fe 4+ 。
更新日期:2024-02-18
中文翻译:

具有异常高价 Fe4+ 的 BaFeO3 多晶型物的氧释放和结合行为
含有异常高价Fe 4+的全氧化钙钛矿BaFeO 3显示出具有相同化学组成的三种晶体多晶型。3C型BaFeO 3具有由共角FeO 6八面体组成的简单立方钙钛矿结构,而6H型和12R型BaFeO 3具有由共角和共面FeO 6八面体组成的六方钙钛矿结构。这些化合物很容易将氧气释放到空气中以还原铁离子的高价态,但氧气释放行为很大程度上取决于晶体结构。3C型BaFeO 3在低至130℃的温度下从FeO 6八面体的角共享位点局部释放氧。相反,6H型和12R型BaFeO 3分别在320℃和460℃以上优先从面共享位释放氧,尽管它们在晶体结构中包括角共享位。所得缺氧的3C型BaFeO 2.5不会将空气中的氧带回,而12R型六方结构则显示出完全可逆的氧释放和空气中的结合。一旦建立了12R型结构,就可以在没有极端条件的情况下稳定异常高价态,例如Fe 4+ 。







































 京公网安备 11010802027423号
京公网安备 11010802027423号