当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peptide-Driven Proton Sponge Nano-Assembly for Imaging and Triggering Lysosome-Regulated Immunogenic Cancer Cell Death
Advanced Materials ( IF 27.4 ) Pub Date : 2024-02-19 , DOI: 10.1002/adma.202307679
Tengyu He 1 , Jing Wen 2 , Wenjian Wang 3 , Zeliang Hu 4 , Chuxuan Ling 5 , Zhongchao Zhao 5 , Yong Cheng 5 , Yu-Ci Chang 1 , Ming Xu 5 , Zhicheng Jin 5 , Lubna Amer 1 , Lekshmi Sasi 5 , Lei Fu 5 , Nicole F Steinmetz 6 , Tariq M Rana 2 , Peng Wu 3 , Jesse V Jokerst 7
Advanced Materials ( IF 27.4 ) Pub Date : 2024-02-19 , DOI: 10.1002/adma.202307679
Tengyu He 1 , Jing Wen 2 , Wenjian Wang 3 , Zeliang Hu 4 , Chuxuan Ling 5 , Zhongchao Zhao 5 , Yong Cheng 5 , Yu-Ci Chang 1 , Ming Xu 5 , Zhicheng Jin 5 , Lubna Amer 1 , Lekshmi Sasi 5 , Lei Fu 5 , Nicole F Steinmetz 6 , Tariq M Rana 2 , Peng Wu 3 , Jesse V Jokerst 7
Affiliation
|
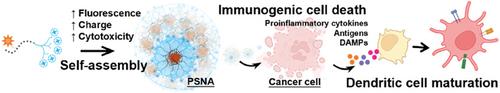
Triggering lysosome-regulated immunogenic cell death (ICD, e.g., pyroptosis and necroptosis) with nanomedicines is an emerging approach for turning an “immune-cold” tumor “hot”—a key challenge faced by cancer immunotherapies. Proton sponge such as high-molecular-weight branched polyethylenimine (PEI) is excellent at rupturing lysosomes, but its therapeutic application is hindered by uncontrollable toxicity due to fixed charge density and poor understanding of resulted cell death mechanism. Here, a series of proton sponge nano-assemblies (PSNAs) with self-assembly controllable surface charge density and cell cytotoxicity are created. Such PSNAs are constructed via low-molecular-weight branched PEI covalently bound to self-assembling peptides carrying tetraphenylethene pyridinium (PyTPE, an aggregation-induced emission-based luminogen). Assembly of PEI assisted by the self-assembling peptide-PyTPE leads to enhanced surface positive charges and cell cytotoxicity of PSNA. The self-assembly tendency of PSNAs is further optimized by tuning hydrophilic and hydrophobic components within the peptide, thus resulting in the PSNA with the highest fluorescence, positive surface charge density, cell uptake, and cancer cell cytotoxicity. Systematic cell death mechanistic studies reveal that the lysosome rupturing-regulated pyroptosis and necroptosis are at least two causes of cell death. Tumor cells undergoing PSNA-triggered ICD activate immune cells, suggesting the great potential of PSNAs to trigger anticancer immunity.
中文翻译:

肽驱动的质子海绵纳米组件用于成像和触发溶酶体调节的免疫原性癌细胞死亡
用纳米药物触发溶酶体调节的免疫原性细胞死亡(ICD,例如细胞焦亡和坏死性凋亡)是一种将“免疫冷”肿瘤变“热”的新兴方法,这是癌症免疫疗法面临的关键挑战。质子海绵如高分子量支化聚乙烯亚胺(PEI)在破坏溶酶体方面表现出色,但由于电荷密度固定且对细胞死亡机制了解不足,其毒性无法控制,阻碍了其治疗应用。在这里,创建了一系列具有自组装可控表面电荷密度和细胞毒性的质子海绵纳米组件(PSNA)。这种 PSNA 是通过低分子量支化 PEI 与携带四苯乙烯吡啶鎓(PyTPE,一种聚集诱导发射的发光剂)的自组装肽共价结合而构建的。自组装肽-PyTPE 辅助 PEI 的组装可增强 PSNA 的表面正电荷和细胞毒性。通过调节肽内的亲水性和疏水性成分,进一步优化 PSNA 的自组装倾向,从而使 PSNA 具有最高的荧光、正表面电荷密度、细胞摄取和癌细胞细胞毒性。系统细胞死亡机制研究表明,溶酶体破裂调节的细胞焦亡和坏死性凋亡至少是细胞死亡的两个原因。接受 PSNA 触发的 ICD 的肿瘤细胞会激活免疫细胞,这表明 PSNA 触发抗癌免疫的巨大潜力。
更新日期:2024-02-19
中文翻译:

肽驱动的质子海绵纳米组件用于成像和触发溶酶体调节的免疫原性癌细胞死亡
用纳米药物触发溶酶体调节的免疫原性细胞死亡(ICD,例如细胞焦亡和坏死性凋亡)是一种将“免疫冷”肿瘤变“热”的新兴方法,这是癌症免疫疗法面临的关键挑战。质子海绵如高分子量支化聚乙烯亚胺(PEI)在破坏溶酶体方面表现出色,但由于电荷密度固定且对细胞死亡机制了解不足,其毒性无法控制,阻碍了其治疗应用。在这里,创建了一系列具有自组装可控表面电荷密度和细胞毒性的质子海绵纳米组件(PSNA)。这种 PSNA 是通过低分子量支化 PEI 与携带四苯乙烯吡啶鎓(PyTPE,一种聚集诱导发射的发光剂)的自组装肽共价结合而构建的。自组装肽-PyTPE 辅助 PEI 的组装可增强 PSNA 的表面正电荷和细胞毒性。通过调节肽内的亲水性和疏水性成分,进一步优化 PSNA 的自组装倾向,从而使 PSNA 具有最高的荧光、正表面电荷密度、细胞摄取和癌细胞细胞毒性。系统细胞死亡机制研究表明,溶酶体破裂调节的细胞焦亡和坏死性凋亡至少是细胞死亡的两个原因。接受 PSNA 触发的 ICD 的肿瘤细胞会激活免疫细胞,这表明 PSNA 触发抗癌免疫的巨大潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号