当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Overlooked Role of Coexistent Hydrogen Peroxide in Activated Peracetic Acid by Cu(II) for Enhanced Oxidation of Organic Contaminants
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-02-15 , DOI: 10.1021/acs.est.3c09753 Jianying Wu 1 , Jing Zou 1 , Jinbin Lin 1, 2 , Sheng Li 1 , Linfeng He 1 , Zhijie Wu 1 , Qingsong Li 3 , Chunming Gong 4 , Jun Ma 5
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-02-15 , DOI: 10.1021/acs.est.3c09753 Jianying Wu 1 , Jing Zou 1 , Jinbin Lin 1, 2 , Sheng Li 1 , Linfeng He 1 , Zhijie Wu 1 , Qingsong Li 3 , Chunming Gong 4 , Jun Ma 5
Affiliation
|
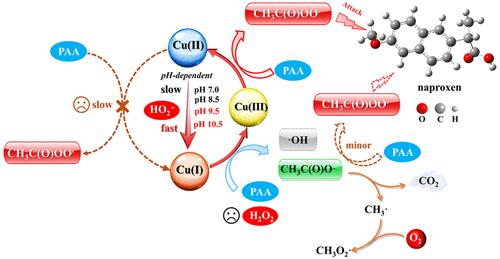
Cu(II)-catalyzed peracetic acid (PAA) processes have shown significant potential to remove contaminants in water treatment. Nevertheless, the role of coexistent H2O2 in the transformation from Cu(II) to Cu(I) remained contentious. Herein, with the Cu(II)/PAA process as an example, the respective roles of PAA and H2O2 on the Cu(II)/Cu(I) cycling were comprehensively investigated over the pH range of 7.0–10.5. Contrary to previous studies, it was surprisingly found that the coexistent deprotonated H2O2 (HO2–), instead of PAA, was crucial for accelerating the transformation from Cu(II) to Cu(I) (kHO2–/Cu(II) = (0.17–1) × 106 M–1 s–1, kPAA/Cu(II) < 2.33 ± 0.3 M–1 s–1). Subsequently, the formed Cu(I) preferentially reacted with PAA (kPAA/Cu(I) = (5.84 ± 0.17) × 102 M–1 s–1), rather than H2O2 (kH2O2/Cu(I) = (5.00 ± 0.2) × 101 M–1 s–1), generating reactive species to oxidize organic contaminants. With naproxen as the target pollutant, the proposed synergistic role of H2O2 and PAA was found to be highly dependent on the solution pH with weakly alkaline conditions being more conducive to naproxen degradation. Overall, this study systematically investigated the overlooked but crucial role of coexistent H2O2 in the Cu(II)/PAA process, which might provide valuable insights for better understanding the underlying mechanism in Cu-catalyzed PAA processes.
中文翻译:

共存过氧化氢在 Cu(II) 活化过乙酸中增强有机污染物氧化作用的被忽视的作用
Cu(II) 催化的过乙酸 (PAA) 工艺已显示出去除水处理污染物的巨大潜力。然而,共存的H 2 O 2在Cu(II) 到Cu(I) 转变中的作用仍然存在争议。本文以Cu(II)/PAA工艺为例,在7.0~10.5的pH范围内全面研究了PAA和H 2 O 2各自对Cu(II)/Cu(I)循环的作用。与之前的研究相反,令人惊讶地发现共存的去质子化 H 2 O 2 (HO 2 – ),而不是 PAA,对于加速从 Cu(II) 到 Cu(I) 的转变至关重要 ( k HO2–/Cu( II) = (0.17–1) × 10 6 M –1 s –1 , k PAA/Cu(II) < 2.33 ± 0.3 M –1 s –1 )。随后,形成的 Cu(I) 优先与 PAA ( k PAA/Cu(I) = (5.84 ± 0.17) × 10 2 M –1 s –1 ) 反应,而不是与 H 2 O 2 ( k H2O2/Cu(I)反应。 ) = (5.00 ± 0.2) × 10 1 M –1 s –1 ),产生活性物质氧化有机污染物。以萘普生为目标污染物,发现H 2 O 2和PAA 的协同作用高度依赖于溶液pH 值,弱碱性条件更有利于萘普生降解。 总体而言,本研究系统地研究了共存的 H 2 O 2在 Cu(II)/PAA 过程中被忽视但至关重要的作用,这可能为更好地理解 Cu 催化 PAA 过程的潜在机制提供有价值的见解。
更新日期:2024-02-15
中文翻译:

共存过氧化氢在 Cu(II) 活化过乙酸中增强有机污染物氧化作用的被忽视的作用
Cu(II) 催化的过乙酸 (PAA) 工艺已显示出去除水处理污染物的巨大潜力。然而,共存的H 2 O 2在Cu(II) 到Cu(I) 转变中的作用仍然存在争议。本文以Cu(II)/PAA工艺为例,在7.0~10.5的pH范围内全面研究了PAA和H 2 O 2各自对Cu(II)/Cu(I)循环的作用。与之前的研究相反,令人惊讶地发现共存的去质子化 H 2 O 2 (HO 2 – ),而不是 PAA,对于加速从 Cu(II) 到 Cu(I) 的转变至关重要 ( k HO2–/Cu( II) = (0.17–1) × 10 6 M –1 s –1 , k PAA/Cu(II) < 2.33 ± 0.3 M –1 s –1 )。随后,形成的 Cu(I) 优先与 PAA ( k PAA/Cu(I) = (5.84 ± 0.17) × 10 2 M –1 s –1 ) 反应,而不是与 H 2 O 2 ( k H2O2/Cu(I)反应。 ) = (5.00 ± 0.2) × 10 1 M –1 s –1 ),产生活性物质氧化有机污染物。以萘普生为目标污染物,发现H 2 O 2和PAA 的协同作用高度依赖于溶液pH 值,弱碱性条件更有利于萘普生降解。 总体而言,本研究系统地研究了共存的 H 2 O 2在 Cu(II)/PAA 过程中被忽视但至关重要的作用,这可能为更好地理解 Cu 催化 PAA 过程的潜在机制提供有价值的见解。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号